ANANDAMIDE
Synonym(s):AEA;Anandamide;Anandamide (20:4, n-6);Arachidonic acid N-(hydroxyethyl)amide
- CAS NO.:94421-68-8
- Empirical Formula: C22H37NO2
- Molecular Weight: 347.53
- MDL number: MFCD00153766
- EINECS: 200-578-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
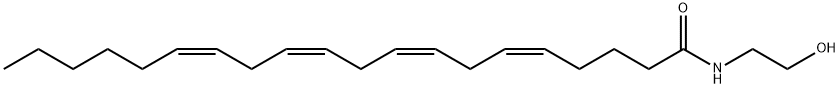
What is ANANDAMIDE?
Description
Anandamide (94421-68-8) is an endogenous cannabinoid receptor agonist.1-3?Ki=89 nM for CB1?and 371 nM for CB2?receptors.
The Uses of ANANDAMIDE
Anandamide is an endogenous cannabinoid and TRPV1 receptor agonist. Findings suggest that endocannabinoids such as anandamide may help cause runner’s high, the feeling of euphoria, reduced anxiety and lessened ability to feel pain associated with exercise.
What are the applications of Application
Anandamide is an endogenous cannabinoid receptor ligand
Definition
ChEBI: An N-(polyunsaturated fatty acyl)ethanolamine resulting from the formal condensation of carboxy group of arachidonic acid with amino group of ethanolamine.
Hazard
A reproductive hazard.
Biological Activity
Endogenous cannabinoid and vanilloid receptor agonist, in water-soluble emulsion (for details see TocrisolveTM 100). K i values are 89 and 371 nM for CB 1 and CB 2 receptors respectively; EC 50 values are 18, 31 and 27 nM at GPR55, CB 1 and CB 2 respectively; pK i = 5.68 for rVR1. Also blocks TNF- α -induced NF-kB activation via direct inhibition of IKK. Also available as the pure oil dissolved in ethanol (N-(2-Hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide ).
Biochem/physiol Actions
An arachidonic acid derivative that is an endogenous ligand for the CB cannabinoid receptor and for the VR1 vanilloid receptor. Inhibits calcium currents in neuroblastomas and neurons. Activates the MAP kinase signaling pathway. Inhibits proliferation and induces apoptosis of lymphocytes and human breast cancer cells.
Enzyme inhibitor
This lipid neurotransmitter and endocannabinoid receptor ligand (FW = 347.54 g/mol; CAS 94421-68-8), also known as arachidonylethanolamide, inhibits calcium ion currents and activates the MAP kinase signaling pathway. Anandamide is a naturally occurring effector of central and peripheral nervous systems, as mediated by CB1 and CB2 cannabinoid receptors, respectively. (These receptors were originally characterized as binding Δ9 -tetrahydrocannabinol, or THC, marijuana’s major psychoactive ingredient.) Anandamide, 2-arachidonoylglycerol, and congeners (N- oleoylethanolamide and N-palmitoylethanolamide) are lipophilic signalling molecules with multiple physiological roles in learning and memory, neuroinflammation, oxidative stress, neuroprotection and neurogenesis. Anandamide likewise plays a role in regulating uterine implantation of the early-stage embryo (blastocyst). Anandamide synthesis occurs from N- acetylphosphatidylethanolamine by multiple pathways that include phospholipase A2, phospholipase C, and NAPE-hydrolyzing phospholipase D (or NAPE-PLD). Target(s): adenylate cyclase; Na + channels; a7-nicotinic acetylcholine receptor; 5 HT3 receptors; Ca 2+ channels; K+ channels, voltage-gated; acylglycerol lipase, or monoacylglycerol lipase.
storage
Store at -20°C
References
1) Devane?et al. (1992),?Isolation and structure of a brain constituent that binds to the cannabinoid receptor; Science,258?1946 2) Devane?et al. (1994),?New dawn of cannabinoid pharmacology; Trends Pharmacol.Sci.,?15?40 3) Hillard?et al. (1997),?Biochemistry and pharmacology of arachidonoylethanolamide, a putative endogenous cannabinoid; J. Lipid Res.,?38?2383
Properties of ANANDAMIDE
| Boiling point: | 522.3±50.0 °C(Predicted) |
| Density | 0.92 g/mL at 25 °C (lit.) |
| Flash point: | 14 °C |
| storage temp. | -20°C |
| solubility | ethanol: soluble |
| form | oil |
| pka | 14.48±0.10(Predicted) |
| color | light yellow |
| Water Solubility | 413ug/L(25 ºC) |
| Stability: | Stable for 1 year from date of purchase as supplied. Subject to oxidation – protect from air. Solutions in degassed DMSO or ethanol may be stored under nitrogen or argon at -80°C for up to 1 month. |
Safety information for ANANDAMIDE
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for ANANDAMIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![(R)-(+)-METHANANDAMIDE [1-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB5728994.gif)

![ANANDAMIDE, [ETHANOLAMINE 1-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB4702050.gif)


![ANANDAMIDE, [ARACHIDONYL-1-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB6403155.gif)
You may like
-
 Arachidonylethanolamide CAS 94421-68-8View Details
Arachidonylethanolamide CAS 94421-68-8View Details
94421-68-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8