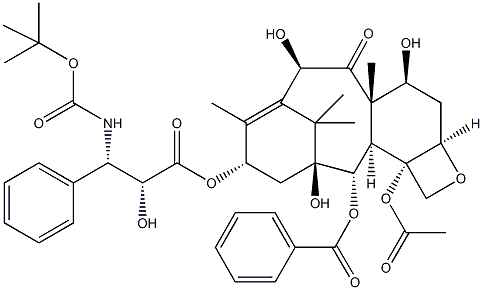
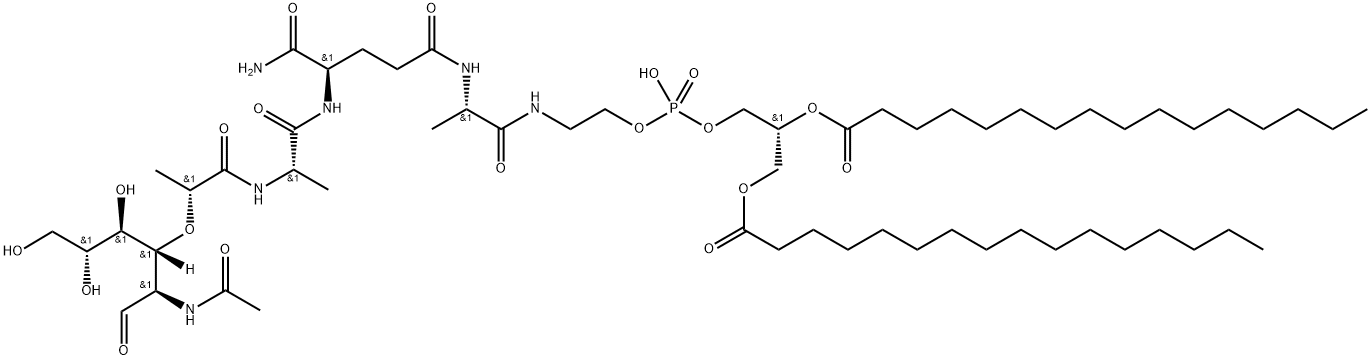
Mifamurtide
Synonym(s):CGP-19835;MTP-cephalin;MTP-PE;Muramyl tripeptide phosphatidylethanolamine;N-(N-Acetylmuramoyl)-L-alanyl-D-α-glutaminyl-N-[(7R)-4-hydroxy-4-oxido-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacos-1-yl]-L-alaninamide
- CAS NO.:83461-56-7
- Empirical Formula: C59H109N6O19P
- Molecular Weight: 1237.52
- MDL number: MFCD09954133
- EINECS: 253-368-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-06 09:45:54
What is Mifamurtide?
Absorption
Due to rapid clearance from plasma, administration of mifamurtide is associated with a very low serum concentration of total (liposomal and free) drug. The mean AUC was 17.0 ± 4.86 h x nM and peak plasma concentration (Cmax) was 15.7 ± 3.72 nM following intravenous administration of 4 mg mifamurtide in healthy adult subjects . Variability in AUC and Cmax is reported to be low .
Toxicity
There have been no reports of overdose with mifamurtide. Signs and symptoms that were associated with higher doses and/or were dose limiting were not life-threatening, and included fever, chills, fatigue, nausea, vomiting, headache and hypotension or hypertension . In cases of suspected overdose, appropriate supportive care is recommended. Gastrointestinal toxicity associated with nausea, vomiting and loss of apetite from mifamurtide therapy is commonly observed.
Mifamurtide was not mutagenic and did not cause teratogenic effects in rats and rabbits. Embryotoxic effects were observed only at maternal toxic levels. There is no evidence of mifamurtide generating harmful effects on male or female reproductive organs. Studies assessing reproductive function, perinatal toxicity and carcinogenicity have not been performed .
The Uses of Mifamurtide
Osteosarcoma.
The Uses of Mifamurtide
Mifamurtide may be used in immunological and cancer-related cell signaling studies.
Indications
Indicated in children, adolescents and young adults for the treatment of high-grade, resectable, non-metastatic osteosarcoma after macroscopically complete surgical resection, typically in combination with post-operative multi-agent chemotherapy .
Background
Mifamurtide is an immunomodulator with antitumor activity via activation of macrophages and monocytes. Also called L-MTP-PE, mifamurtide may be a liposomal form of of the active ingredient MTP-PE, which is a synthetic, less pyrogenic, and longer-acting derivative of muramyl dipeptide (MDP). MDP is a motif present in all gram-positive and gram-negative bacterial walls that is recognized by different signalling molecules and activators such as nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) and toll-like receptors present in macrophages and monocytes. The overall result of MDP recognition leads to the production of proinflammatory cytokines and promotion of bactericidal and tumoricidal effects . As a liposomal formulation, mifamurtide demonstrates an enhanced tumoricidal effect and improved safety profile .
Mifamurtide is marketed in Europe as Mepact for intravenous infusion. It is administered as an adjuvant therapy to postoperative combination chemotherapy in pediatric, adolescent or adult patients with high-grade, resectable, non-metastatic osteosarcoma after macroscopically complete surgical resection. In the US, it is currently under investigation that holds orphan drug status for the treatment of osteosarcoma .
Osteosarcoma is the most common primary malignant bone tumor that usually arises in the metaphyses of long bone in children and adolescents . The standard therapy for osteosarcoma is comprised of macroscopic surgical resection and multi-agent chemotherapy consisting of doxorubicin, cisplatin, high-dose methotrexate with leucovorin rescue, and ifosfamide . While about 90% of patients with newly diagnosed osteosarcoma may achieve complete remission from first-line therapies, the prognosis is still poor for patients with non-metastatic osteosarcoma with lower 5-year event-free survival. In a large, randomized, open-label, multicenter, phase III trial, the treatment of mifamurtide in conjunction with three- or four-drug combination chemotherapy (doxorubicin, cisplatin, and high-dose methotrexate with, or without, ifosfamide) was associated with significant improvement in survival rates and good tolerance . The adverse events (AEs) associated with mifamurtide were generally mild to moderate in severity .
Biochem/physiol Actions
Mifamurtide is an immunomodulator and regulates the activation of monocytes and macrophages. Mifamurtide upregulates the secretion of pro-inflammatory cytokines such as TNF-α, IL-1, IL-8, nitric oxide and prostaglandins E2 and D2. It has anti-tumor effects in children and young adults with high-grade osteosarcoma.
Mechanism of action
Being a phospholipid, mifamurtide accumulates in the lipid bilayer of the liposomes upon infusion. After application of the liposomal infusion, the drug is cleared from the plasma within minutes. However, it is concentrated in lung, liver, spleen, nasopharynx and thyroid, and the terminal half-life is 18 hours, which is longer than the natural substance.
Pharmacokinetics
Mifamurtide stimulates the innate immunity by activating monocytes and macrophages. Within hours following administration of mifamurtide in healthy adults or patients with osteosarcoma NOS, elevated plasma levels of proinflammatory molecules, such as TNF-α, IL-6, and IL-1β, and other indicators of immune stimulation like C-reactive protein and neopterine were observed . In vivo administration of mifamurtide in rat and mouse model resulted in inhibition of tumour growth of lung metastasis, skin and liver cancer, and fibrosarcoma . In addition, increased disease-free survival rate was demonstrated when mifamurtide was given as an adjuvant in dog models of osteosarcoma and hemangiosarcoma. Administration of mifamurtide was associated with transient neutropenia, usually when used in conjunction with chemotherapy. Pronounced inflammatory responses are uncommon .
Clinical Use
Mifamurtide is an anticancer agent for the treatment of osteosarcoma, the most common primary malignancy of bone tissue mainly affecting children and adolescents. The drug was invented by Ciba-Geigy (now Novartis) in the early 1980s and the agent was subsequently licensed to Jenner Biotherapies in the 1990s. IDM Pharma bought the rights to the drug from Jenner in April 2003.In March 2009, mifamurtide was approved in the 27 European Union member states plus Iceland, Liechtenstein and Norway via a centralized marketing authorization. After the approval, IDM Pharma was acquired by Takeda, which began launching mifamurtide, as Mepact ®, in February 2010. Mifamurtide, a fully synthetic lipophilic derivative of muramyl dipeptide (MDP), is muramyl tripeptide phosphatidylethanolamine (MTP-PE), which is formulated as a liposomal infusion.
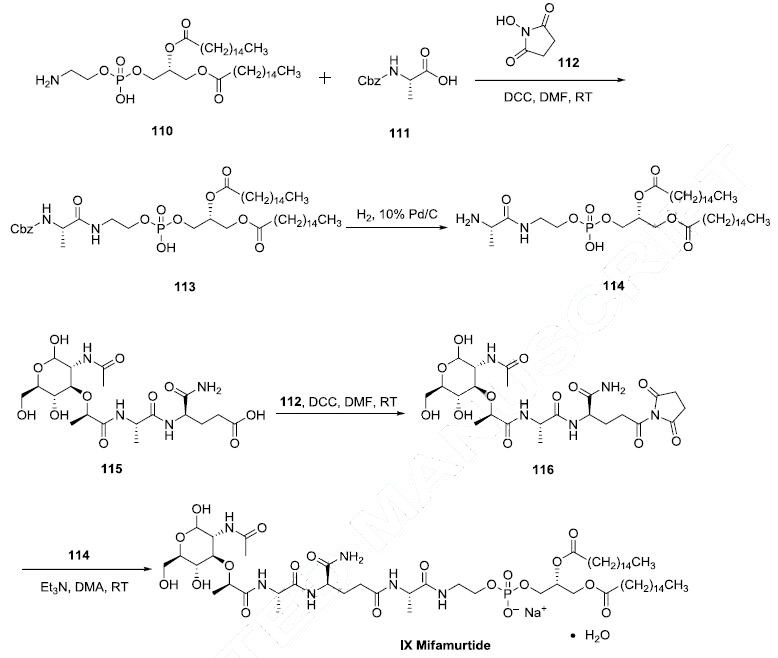
Synthesis
Two synthetic routes have been reported, and the scheme describes the more process-amenable route. Commercially available 1,2-dipalmitoyl-snglycero- 3-phosphoethanolamine (110) was coupled with N-Boc-L-alanine (111) by means of Nhydroxysuccinimide (112), DCC in DMF to give amide 113, which was followed by hydrogenolysis of the CBZ group to give the corresponding L-alanyl-phosphoric acid 114. Next, commercially available N-acetylmuramoyl-L-alanyl-D-isoglutamine (115) was subjected to hydroxybenzotriazole (HOBT) and DIC in DMF to provide the corresponding succinimide ester 116 which was condensed with compound 114 to provide mifamurtide (IX). No yields were provided for these transformations.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: avoid with high dose NSAIDs.
Ciclosporin: avoid concomitant use.
Corticosteroids: avoid concomitant use.
Tacrolimus: avoid concomitant use.
Metabolism
Metabolism of liposomal MTP-PE has not been studied in humans . The liposomes are mainly phagocytosed by the cells of the reticuloendothelial system (RES) .
Metabolism
The cells of the reticuloendothelial system clear mifamurtide liposomes by phagocytosis.
Properties of Mifamurtide
| Density | 1.152 |
| storage temp. | -20°C |
| solubility | water: soluble2mg/mL, clear (warmed) |
| form | powder |
| pka | 1.39±0.50(Predicted) |
| color | white to beige |
| CAS DataBase Reference | 83461-56-7 |
Safety information for Mifamurtide
Computed Descriptors for Mifamurtide
| InChIKey | ZVLWUMPAHCEZAW-HYGHKABSNA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1