Moxaverine hydrochloride
- CAS NO.:1163-37-7
- Empirical Formula: C20H22ClNO2
- Molecular Weight: 343.85
- MDL number: MFCD00800728
- EINECS: 214-607-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-09-03 12:56:46
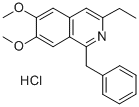
What is Moxaverine hydrochloride?
Originator
Paverin,Bracco
The Uses of Moxaverine hydrochloride
Moxaverine Hydrochloride is used as a nanocomposites as drug-carriers.
Manufacturing Process
166 g (1 mol) of 3,4-dimethoxybenzaldehyde were stirred for several hours (8
to 10 hours) with 180 g (2.02 mols.) of 1-nitropropane in 300 ml of methanol,
in which 12 g of metallic sodium had previously been dissolved, the stirring
taking place while heating to 45-50°C. After usual working up of the reaction mixture, there were obtained 155 g of a white, crystalline product, which
constituted the 1-(3,4-dimethoxyphenyl)-2-nitro-1-butanol and melted after
recrystallization from isopropanol at 93-94°C (uncorrected). The composition
was confirmed by elementary analysis and an infra-red spectrogram.
204 g (0.8 mol) of the above nitro alcohol were reduced at 30-35°C in 1250 g
of 44% formic acid with 320 g of powdered zinc (about 4.9 at.). After working
up, the base 1-(3,4-dimethoxyphenyl)-2-amino-1-butanol was obtained as a
white crystalline product. After recrystallization from ethyl acetate, it melted
at 91-93°C and the yield was 168 g, i.e. 93.3% of the theoretical.
90 g (0.4 mol) of the above amino alcohol are reacted at 45-50°C in 400 ml
of chloroform in the presence of 95 g (1.2 mols) of pyridine by means of 139
g (0.9 mol) of phenylacetic acid chloride. After working up the reaction
mixture, a yellowish-crystalline product was isolated (183.5 g, theoretical:
184.6 g), which melted at 123-125°C. After recrystallization from ethyl
acetate, it yielded minute, white crystals, which melted at 129 -131°C
(uncorrected). The composition of the product was confirmed by elementary
analysis.
217 g of the above 1-(3,4-dimethoxyphenyl)-2-(phenylacetamido)-butanol-1-
phenyl acetate (0.47 mol) were stirred in 80 1300 ml of xylene with 145 g of
phosphorous oxychloride at 100-105°C. After some hours, when the evolution
of hydrochloric acid gas had ceased, the reaction mixture was poured on to
ice and stirred while cold until the crystallization was completed. After
filtering, 144 g (89%) of the formed 1-benzyl-6,7-dimethoxy-3-ethyl
isoquinoline hydrochloride were obtained in the form of yellowish crystals,
which melted at 198-202°C with decomposition. From the separated aqueous
mother liquors, the remainder of the formed isoquinoline base was obtained
after treatment with ammonia and extraction with ether, the said base being
isolated by way of the sparingly soluble and readily crystallisable acid sulfate.
The salt represented a yellowish crystal powder, which melted at 239-243°C
and weighed 21 g (11%). Thus, the yield of crude isoquinoline salt was almost
the theoretical yield. The crude hydrochloride acid salt yielded white, lustrous
prisms after recrystallisation from 96% ethanol, the said prisms melting at
208-210°C with decomposition. 1-Benzyl-6,7 -dimethoxy-3-ethyl isoquinoline
may be prepared as a base from its salt by adding of equivalent of triethyl
amine or any other base.
Therapeutic Function
Spasmolytic
Properties of Moxaverine hydrochloride
| Melting point: | 208-210° (dec) |
Safety information for Moxaverine hydrochloride
Computed Descriptors for Moxaverine hydrochloride
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4