Monoperoxyphthalic acid magnesium salt hexahydrate
Synonym(s):Magnesium bis(monoperoxyphthalate) hexahydrate;MMPP
- CAS NO.:84665-66-7
- Empirical Formula: C16H11MgO11-
- Molecular Weight: 403.56
- MDL number: MFCD00149614
- EINECS: 617-608-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-06 16:16:43
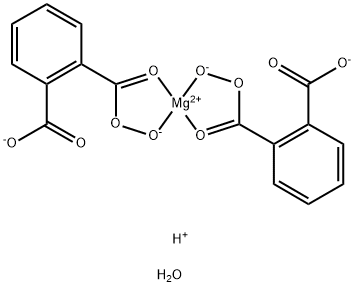
What is Monoperoxyphthalic acid magnesium salt hexahydrate?
Chemical properties
White Granules
The Uses of Monoperoxyphthalic acid magnesium salt hexahydrate
Monoperoxyphthalic acid magnesium salt hexahydrate is used as disinfectant for hard surfaces (hospital, food industrial, institution).
The Uses of Monoperoxyphthalic acid magnesium salt hexahydrate
Reagent for:
- Trans-diaxial hydroxylation under bismuth(III) triflate catalyst
- Oxidation rections under mild conditions
- Epoxidation of unsaturated steroids
- Synthesis of ring-B oxygenated steroids as anticancer agents
What are the applications of Application
Magnesium monoperoxyphthalate hexahydrate is a mild, general oxidizing agent
General Description
Magnesium monoperoxyphthalate hexahydrate is an organic peroxide used in many oxidation reactions. It exhibits non-deflagrating and non-shock-sensitive properties and also used as a surface disinfectant.
Purification Methods
MMPP is a safer reagent than m-chloroperbenzoic acid for oxidation reactions because it is not as explosive and has advantages of solubility. It is soluble in H2O, low-molecular-weight alcohols, i-PrOH and DMF. The product of reaction, Mg phthalate, is soluble in H2O. It has been used in aqueous phase to oxidise compounds in e.g. CHCl3 and using a phase transfer catalyst, e.g. methyltrioctylammonium chloride [Brougham Synthesis 1015 1987]. The oxidising activity can be checked (as for perbenzoic acid in Silbert et al. Org Synth Coll Vol V 906 1973], and if found to be low it would be best to prepare afresh from phthalic anhydride (1mol), H2O2 (1mol) and MgO at 20-25o to give MMPP. [Hignett, European Pat Appl 27 693 1981, Chem Abstr 95 168810 1981, Beilstein 9 IV 3260.]
Properties of Monoperoxyphthalic acid magnesium salt hexahydrate
| Melting point: | 93 °C (dec.)(lit.) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO, Methanol |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 84665-66-7(CAS DataBase Reference) |
Safety information for Monoperoxyphthalic acid magnesium salt hexahydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H312:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Monoperoxyphthalic acid magnesium salt hexahydrate
| InChIKey | WWOYCMCZTZTIGU-UHFFFAOYSA-L |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

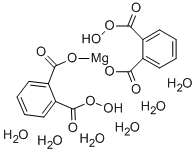
You may like
-
 Magnesium monoperoxyphthalate hexahydrate CASView Details
Magnesium monoperoxyphthalate hexahydrate CASView Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8