Monoacetin
- CAS NO.:26446-35-5
- Empirical Formula: C5H10O4
- Molecular Weight: 134.13
- MDL number: MFCD00036185
- EINECS: 247-704-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
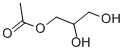
What is Monoacetin?
Chemical properties
Clear colorless to pale yellow viscous liquid
The Uses of Monoacetin
Glycerol monoacetate is used for production of tanning leather and dye. It is also used as a solvent.
The Uses of Monoacetin
In manufacture of smokeless powder and dynamite; as a solvent for basic dyes; in tanning leather.
What are the applications of Application
Monoacetin has found some use as a flavor solvent (rarely as a perfume solvent).
Production Methods
Monoacetin is manufactured by heating glycerol with acetic acid.
Synthesis Reference(s)
Journal of the American Chemical Society, 82, p. 4883, 1960 DOI: 10.1021/ja01503a033
General Description
Clear colorless to pale yellow viscous liquid with a characteristic odor.
Air & Water Reactions
Very hygroscopic. Soluble in water.
Reactivity Profile
Monoacetin may hydrolyze in acid or alkaline aqueous solutions. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. They exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Fire Hazard
Monoacetin is probably combustible.
Properties of Monoacetin
| Melting point: | 3 °C |
| Boiling point: | 258 °C |
| Density | 1.21 |
| Flash point: | 145 °C |
| refractive index | 1.4500 |
| Specific Gravity | 1.2060 (20℃) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,6242 |
| Exposure limits | OSHA: TWA 15 mg/m3; TWA 5 mg/m3 |
| CAS DataBase Reference | 26446-35-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Monoacetine(26446-35-5) |
| EPA Substance Registry System | Glyceryl monoacetate (26446-35-5) |
Safety information for Monoacetin
Computed Descriptors for Monoacetin
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1