Molybdenum trioxide
Synonym(s):Molybdenum trioxide;Molybdenum trioxide, Molybdic acid anhydrous, Molybdic anhydride;Molybdenum(VI) oxide
- CAS NO.:1313-27-5
- Empirical Formula: MoO3
- Molecular Weight: 143.94
- MDL number: MFCD00003469
- EINECS: 215-204-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-01 18:33:23
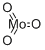
What is Molybdenum trioxide?
Description
Molybdenum trioxide is an odorless, whitecrystalline powder that turns yellow when heated; Freezing/Melting point 5 795℃. Hazard Identification (based onNFPA-704 M Rating System): Health 2, Flammability 0,Reactivity 0. Slightly soluble in water.
Chemical properties
Molybdenum trioxide is an odorless, white crystalline powder that turns yellow when heated.

Molybdenum trioxide is perhaps the most important compound of molybdenum. Pure molybdenum trioxide is used in chemical and catalyst manufacture. The technical product is added to steel as an alloying agent. Molybdenum trioxide also serves as a catalyst in the petroleum industry and as a component of ceramics, enamels and pigments.
Physical properties
Soft white powder; orthorhombic crystals; turns yellow on heating; density 4.69 g/cm3 at 21°C; melts at 795°C without decomposition to a dark yellow liquid; vapor pressure 20 torr at 851°C and 200 torr at 1,014°C; boils at 1,155°C; sparingly soluble in cold water (1.066 g/L at 18°C) and moderately soluble in hot water (20.55 g/L at 70°C); dissolves in acids and alkalies.
The Uses of Molybdenum trioxide
Molybdenum trioxide (MnO3) is a compound used to make enamels adhere to metals.
The Uses of Molybdenum trioxide
Molybdenum(VI) oxide is used in catalyst compositions to carry out desulfurization of petroleum feedstocks and to remove nitrogen-containing compounds from petroleum fractions. Other uses of this oxide include preparation of various molybdate salts and as reagents for chemical analyses.
The Uses of Molybdenum trioxide
Chiefly as a reagent for chemical analysis.Used for trace metal analysis.
What are the applications of Application
Molybdenum(VI) oxide is a chemical used in the solid state synthesis of a reduced molybdenum oxide
Preparation
Molybdenum(VI) oxide is obtained by igniting molybdenum or its compounds in air:
2Mo + 3O2 → 2MoO3
2MoS2 + 7O2 → 2MoO3 + 4SO2
MoS2 + 4O2 → MoO3 + SO2 + SO3
Roasting the sulfide is carried out in a multiple-hearth roaster under controlled temperature and airflow. The product mixture is sublimed to obtain high purity oxide.
Purified molybdenum(VI) oxide also is made by prolonged heating of ammonium molybdate in air:
(NH4)2Mo2O7 → 2MoO3 + 2NH3 + H2O
An alternative method involves repeatedly evaporating a mixture of ammonium molybdate and nitric acid. Ammonium nitrate so formed is separated from the product molybdenum(VI) oxide by extraction with water:
(NH4)2Mo2O7 + 2HNO3 → 2MoO3 + 2NH4NO3 + H2O
Definition
ChEBI: Molybdenum trioxide is a molybdenum oxide.
General Description
Colorless to white or yellow odorless solid. Sinks in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Molybdenum trioxide reacts violently with chlorine trifluoride, lithium, potassium and sodium. Readily combines with acids and bases to form a series of polymeric compounds. Incompatible with interhalogens and metals. A violent detonation occurs when heated with molten magnesium.
Hazard
Toxic material.
Health Hazard
Compound is relatively nontoxic. Dust irritates eyes.
Fire Hazard
Flash point data for Molybdenum trioxide are not available, but Molybdenum trioxide is probably non-flammable.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion, subcutaneous, and intraperitoneal routes. Human systemic effects by inhalation: pulmonary fibrosis and cough. Questionable carcinogen with experimental neoplastigenic data. A powerful irritant. Explodes on contact with molten magnesium. Violent reaction with interhalogens (e.g., bromine pentafluoride, chlorine trifluoride). Incandescent reaction with hot sodium, potassium, or lithium. When heated to decomposition it emits toxic fumes of Mo.
Potential Exposure
Molybdenum trioxide is used in agriculture; manufacture of metallic molybdenum, ceramic glazes; enamels, pigments, and in analytical chemistry.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Molybdenum trioxide must be stored to avoid contact with strong acids (such as hydrochloric, sulfuric, andnitric); alkalis, sodium, potassium, and molten magnesiumsince violent reactions occur. Store in tightly closed containers in a cool, well-ventilated area.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise it from water (1g/50mL) between 70o and 0o. The solubility in H2O is 0.1% at 18o, and 2% at 70o. It is a white powder which turns yellow reversibly on heating. It sublimes readily at 1155o/760mm. [Hein & Herzog Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1412 1965.]
Incompatibilities
Explodes on contact with molten magnesium. Violent reaction with strong oxidizers, such as chlorine trifluoride; bromine pentafluoride. Not compatible with strong acids; active metals (sodium, potassium, lithium).
Properties of Molybdenum trioxide
| Melting point: | 795 °C(lit.) |
| Boiling point: | 1155 °C |
| Density | 4.692 |
| vapor pressure | 0Pa at 20℃ |
| Flash point: | 1155°C subl. |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 0.49g/l |
| form | Sublimed Powder |
| Specific Gravity | 4.69 |
| color | White to gray |
| Water Solubility | 0.5 g/L (20 ºC) |
| Merck | 14,6239 |
| Exposure limits | ACGIH: TWA 10 mg/m3; TWA 3 mg/m3 NIOSH: IDLH 5000 mg/m3 |
| CAS DataBase Reference | 1313-27-5(CAS DataBase Reference) |
| IARC | 2B (Vol. 118) 2018 |
| NIST Chemistry Reference | Molybdenum(vi) oxide(1313-27-5) |
| EPA Substance Registry System | Molybdenum trioxide (1313-27-5) |
Safety information for Molybdenum trioxide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Molybdenum trioxide
| InChIKey | JKQOBWVOAYFWKG-UHFFFAOYSA-N |
Molybdenum trioxide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Molybdenum Trioxide 98%View Details
Molybdenum Trioxide 98%View Details -
 Molybdenum trioxide 99%View Details
Molybdenum trioxide 99%View Details -
 Molybdenum(VI) oxide CAS 1313-27-5View Details
Molybdenum(VI) oxide CAS 1313-27-5View Details
1313-27-5 -
 Molybdenum(VI) oxide CAS 1313-27-5View Details
Molybdenum(VI) oxide CAS 1313-27-5View Details
1313-27-5 -
 Molybdenum(VI) oxide CAS 1313-27-5View Details
Molybdenum(VI) oxide CAS 1313-27-5View Details
1313-27-5 -
 Molybdenum(VI) oxide CAS 1313-27-5View Details
Molybdenum(VI) oxide CAS 1313-27-5View Details
1313-27-5 -
 Molybdenum(VI) oxide CAS 1313-27-5View Details
Molybdenum(VI) oxide CAS 1313-27-5View Details
1313-27-5 -
 Molybdenum(VI) oxide CAS 1313-27-5View Details
Molybdenum(VI) oxide CAS 1313-27-5View Details
1313-27-5
