Moexipril
- CAS NO.:103775-10-6
- Empirical Formula: C27H34N2O7
- Molecular Weight: 498.57
- MDL number: MFCD00865878
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
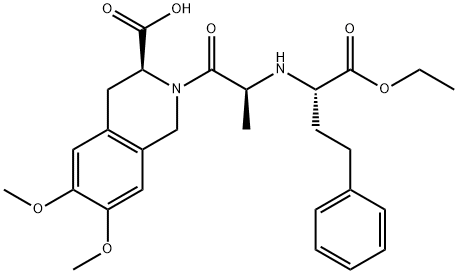
What is Moexipril?
Absorption
Moexipril is incompletely absorbed, with bioavailability as moexiprilat of about 13% compared to intravenous (I.V.) moexipril (both measuring the metabolite moexiprilat), and is markedly affected by food, which reduces Cmax and AUC by about 70% and 40%, respectively, after the ingestion of a low-fat breakfast or by 80% and 50%, respectively, after the ingestion of a high-fat breakfast.
Toxicity
Human overdoses of moexipril have not been reported. In case reports of overdoses with other ACE inhibitors, hypotension has been the principal adverse effect noted. Single oral doses of 2 g/kg moexipril were associated with significant lethality in mice. Rats, however, tolerated single oral doses of up to 3 g/kg. Common adverse effects include cough, dizziness, diarrhea, flu syndrome, fatigue, pharyngitis, flushing, rash, and myalgia
Originator
Moexipril,Schwarz Pharma,Germany
The Uses of Moexipril
Antihypertensive; enzyme inhibitor (angiotensinconverting).
Background
Moexipril is a non-sulfhydryl containing precursor of the active angiotensin-converting enzyme (ACE) inhibitor moexiprilat. It is used to treat high blood pressure (hypertension). It works by relaxing blood vessels, causing them to widen. Lowering high blood pressure helps prevent strokes, heart attacks and kidney problems.
Indications
For the treatment of hypertension.
What are the applications of Application
moexipril is a potent ACE inhibitor
Definition
ChEBI: Moexipril is a peptide.
Manufacturing Process
1) A solution of 2.0 g of t-butyl alanine (S-form) and 3.78 g of ethyl 2-bromo-
4-phenylbutanoate in 25 ml of DMF was treated with 1.8 ml of triethylamine
and the solution was heated at 70°C for 18 hours. The solvent was removed
at reduced pressure and the residue was mixed with water and extracted with
ethyl ether. The organic layer was washed with water and dried over
magnesium sulfate. Concentration of the solvent at reduced pressure gave the
oily ethyl-α-[(1-carboxyethyl)amino]benzene-t-butanoate.
A solution of 143.7 g of this t-butyl ester in 630 ml of trifluoroacetic acid was
stirred at room temperature for one hour. The solvent was removed at
reduced pressure and the residue was dissolved in ethyl ether and again
evaporated. This operation was repeated. Then the ether solution was treated
dropwise with a solution of hydrogen chloride gas in ethyl ether until
precipitation ceased. The solid, collected by filtration, was a mixture of
diastereoisomers of ethyl-α-[(1-carboxyethyl)amino]benzenebutanoate
hydrochloride, melting point 153-165°C; [α]D23 = +3.6° (1% MeOH).
The free amino acid (S,S-form) was prepared by treatment of an aqueous
solution of the hydrochloride with saturated sodium acetate. The product was
filtered, washed efficiently with cold water and recrystallized from ethyl
acetate; melting point 149-151°C; [α]D23 = +29.7°.
2) A stirred solution of 0.0158 mole of ethyl-α-[(1-carboxyethyl)amino]
benzenebutanoate hydrochloride in 200 ml of methylene chloride was treated
successively with 1.60 g (0.0158 mole) of triethylamine, 0.0158 mole of 1-
hydroxybenzotriazole, 0.0158 mole of 1,2,3,4-tetrahydro-6,7-dimethoxy-3-
isoquinolinecarboxylic acid and then with 0.0158 mole of
dicyclohexylcarbodiimide in 10 ml of methylene dichloride. Dicyclohexylurea
gradually separated. The mixture was allowed to stand at room temperature
overnight. Hexane (300 ml) was added and the urea was filtered. The filtrate
was washed with 250 ml of saturated sodium bicarbonate, dried over sodium
sulfate and concentrated to remove solvent. The viscous residue was
triturated with 50 ml of ether and filtered to remove insolubles. The filtrate
was concentrated to give 2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-
oxopropyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-3-isoquinolinecarboxylic acid.
After addition of hydrochloric acid was obtained 2-[2-[[1-(ethoxycarbonyl)-3-
phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-3-
isoquinolinecarboxylic acid, hydrochloride.
brand name
Univasc (Schwarz Pharma).
Therapeutic Function
Antihypertensive
Pharmacokinetics
Moexipril is a non-sulfhydryl containing precursor of the active angiotensin-converting enzyme (ACE) inhibitor moexiprilat. It is used to treat high blood pressure (hypertension). It works by relaxing blood vessels, causing them to widen. Lowering high blood pressure helps prevent strokes, heart attacks and kidney problems.
Metabolism
Rapidly converted to moexiprilat, the active metabolite. Conversion to the active metabolite is thought to require carboxyesterases and is likely to occur in organs or tissues, other than the gastrointestinal tract, in which carboxyesterases occur. The liver is thought to be one site of conversion, but not the primary site.
Properties of Moexipril
| Boiling point: | 709.3±60.0 °C(Predicted) |
| Density | 1?+-.0.06 g/cm3(Predicted) |
| pka | 2.94±0.20(Predicted) |
| CAS DataBase Reference | 103775-10-6(CAS DataBase Reference) |
Safety information for Moexipril
Computed Descriptors for Moexipril
Moexipril manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 103775-10-6 Moexipril 98%View Details
103775-10-6 Moexipril 98%View Details
103775-10-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8