Tamoxifen citrate
Synonym(s):TMX;TXNDC;TXNDC1;(Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene;Estrogen Receptor Signaling Regulator I
- CAS NO.:54965-24-1
- Empirical Formula: C32H37NO8
- Molecular Weight: 563.64
- MDL number: MFCD00058321
- EINECS: 259-415-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-30 17:36:11
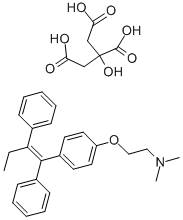
What is Tamoxifen citrate?
Description
Tamoxifen citrate (54965-24-1) is an estrogen receptor antagonist/partial agonist. Induces oxidative stress and apoptosis in estrogen receptor-negative cancer cell lines.1 Displays neuroprotective effects in permanent focal ischemia.2 Inhibits PKC.3 Potent agonist at GPR30 (membrane estrogen receptor).4 Clinically useful breast cancer agent.5
Chemical properties
Tamoxifen citrate is a white to off-white powder with no smell. It can dissolve in methanol, ethanol, and acetone to varying degrees and can slightly dissolve in trichloromethane, but it is almost insoluble in water. It can dissolve in glacial acetic acid. When tamoxifen citrate reaches a temperature between 142-148°C, it melts and decomposes at the same time.
Originator
Nolvadex,I.C.I.,UK,1973
The Uses of Tamoxifen citrate
Tamoxifen Citrate is a selective estrogen response modifier (SERM), protein kinase C inhibitor and anti-angiogenetic factor. Tamoxifen is a prodrug that is metabolized to active metabolites 4-hydroxytamoxifen (4-OHT) and endoxifen by cytochrome P450 isoforms CYP2D6 and CYP3A4.
The Uses of Tamoxifen citrate
Tamoxifen citrate is commonly used in the treatment of women with metastatic carcinoma of the breast to prevent recurrence after surgery or radiation treatment, and as prophylaxis for patients at very high risk to develop breast cancer.
The Uses of Tamoxifen citrate
anti-estrogen, protein kinase C inhibitor, beneficial cardiovascular effects, bone cancer treatment, induces DNA adduct formation
What are the applications of Application
Tamoxifen Citrate is an antiestrogen with potent anti-cancer and PKC inhibitory activities
Manufacturing Process
To the Grignard reagent prepared from 0.59 part of magnesium, 3.95 parts of
bromobenzene and 50 parts of ether there are added 7.5 parts of 4-(β-
dimethylaminoethoxy)-α-ethyldesoxybenzoin in 50 parts of ether. After
heating under reflux for 3 hours, the mixture is decomposed by the addition
of a solution of 60 parts of ammonium chloride in 150 parts of water. The
mixture is separated, and the ethereal layer is dried with anhydrous sodium
sulfate, and the ether is evaporated. The residue is crystallized from
methanol. There is thus obtained 1-(p-β-dimethylaminoethoxyphenyl)-1,2-
diphenylbutan-1-ol, melting point 120°C to 121°C.
2.15 parts of 1-(p-β-dimethylaminoethoxyphenyl)-1,2-diphenylbutan-1-ol, 25
parts of ethanol and 0.8 part of 10 N hydrochloric acid are heated together
under reflux for 3 hours. The solution is evaporated to dryness under reduced
pressure and the residue is extracted with methylene chloride. The methylene
chloride extract is decolorized with charcoal and then evaporated to dryness.
The residue is dissolved in 100 parts of water, the solution is basified by the
addition of sodium hydroxide solution, and the precipitated solid is extracted
three times, each time with 50 parts of ether. The combined extracts are dried with anhydrous sodium sulfate and then evaporated. The residue is
crystallized from aqueous methanol, and there is thus obtained 1-(p-β-
dimethylaminoethoxyphenyl)-1,2-diphenylbut-1-ene, melting point 95°C to
96°C.
brand name
Nolvadex (AstraZeneca); Soltamox (Savient).
Therapeutic Function
Antiestrogen, Antineoplastic
General Description
Tamoxifen, 2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-N,N-dimethylethanamine(Nolvadex), is a triphenylethylene SERM used to treatearly and advanced breast carcinoma in postmenopausalwomen. Tamoxifen is used as adjuvant treatment for breastcancer in women following mastectomy and breast irradiation.It reduces the occurrence of contralateral breast cancerin patients receiving adjuvant tamoxifen therapy. It is alsoeffective in the treatment of metastatic breast cancer in bothwomen and men. In premenopausal women with metastaticbreast cancer, tamoxifen is an alternative to oophorectomyor ovarian irradiation. Tamoxifen can be used preventativelyto reduce the incidence of breast cancer in women athigh risk. Antiestrogenic and estrogenic side effects caninclude hot flashes, nausea, vomiting, platelet reduction,and (in patients with bone metastases) hypercalcemia. Likeall triphenylethylene derivatives, it should be protectedfrom light.
Biological Activity
Estrogen receptor antagonist/partial agonist. Selective and potent inhibitor of mammalian sterol isomerase. Neuroprotective in female rats in vivo . Also high affinity agonist at the membrane estrogen receptor GPR30.
Biochem/physiol Actions
Cell permeable: yes
Safety Profile
Confirmed human carcinogen with experimental carcinogenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Experimental reproductive effects. Human systemic effects: visual field changes, retinal changes. An anti-estrogenic drug. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
The major metabolite of tamoxifen is N-desmethyltamoxifen,which reaches steady-state levels higher than tamoxifenitself. It is believed that N-desmethyltamoxifen contributessignificantly to the overall antiestrogenic effect. Anothermetabolite, 4-hydroxytamoxifen, is a more potent antiestrogenthan tamoxifen, but because it is only a minor metaboliteof tamoxifen, it probably does not contribute significantly tothe therapeutic effects. 4-Hydroxytamoxifen, with its greateraffinity for the ERs, however, has been used extensively inpharmacological studies of these receptors. Tamoxifen concentrationsare reduced if coadministered with rifampin, a cytochromeP450 inducer.
Storage
+4°C
References
1) Ferlini et al. (1999), Tamoxifen induces oxidative stress and apoptosis in oestrogen receptor-negative human cancer cell lines; Br. J. Cancer, 79 257
2) Kimelberg et al. (2003), Neuroprotective activity of tamoxifen in permanent focal ischemia; J. Neurosurg., 99 138
3) Couldwell et al. (1994), Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines; FEBS Lett., 345 43
4) Thomas et al. (2005), Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells; Endocrinology, 146 624
5) Jordan et al. (2006), Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer; Br. J. Pharmacol., 147 S269
Properties of Tamoxifen citrate
| Melting point: | 140-144 °C |
| storage temp. | 2-8°C |
| solubility | methanol: soluble50mg/mL, clear, colorless |
| form | Powder |
| color | White to off-white |
| Water Solubility | slightly soluble |
| Sensitive | Light Sensitive & Hygroscopic |
| Merck | 14,9048 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
| CAS DataBase Reference | 54965-24-1(CAS DataBase Reference) |
| EPA Substance Registry System | Tamoxifen citrate (54965-24-1) |
Safety information for Tamoxifen citrate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Tamoxifen citrate
| InChIKey | FQZYTYWMLGAPFJ-OQKDUQJOSA-N |
| SMILES | C(/C1=CC=CC=C1)(\C1C=CC(OCCN(C)C)=CC=1)=C(/CC)\C1=CC=CC=C1.C(O)(C(=O)O)(CC(=O)O)CC(=O)O |
Tamoxifen citrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![alpha-[4-[2-(dimethylamino)ethoxy]phenyl]-beta-ethyl-alpha-phenylphenethyl alcohol](https://img.chemicalbook.in/CAS/GIF/748-97-0.gif)
![2-[4-[(E)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethyl-ethanamine](https://img.chemicalbook.in/CAS/GIF/13002-65-8.gif)
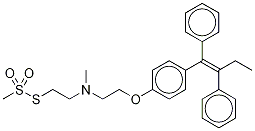
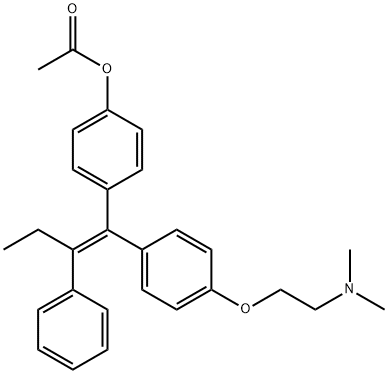
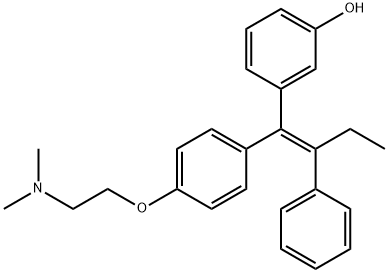
You may like
-
 Tamoxifen citrate 98.00% CAS 54965-24-1View Details
Tamoxifen citrate 98.00% CAS 54965-24-1View Details
54965-24-1 -
 Tamoxifen Citrate IPView Details
Tamoxifen Citrate IPView Details
10540-29-1 -
 99% Tamoxifen Citrate Api Powder manufacturer indiaView Details
99% Tamoxifen Citrate Api Powder manufacturer indiaView Details
54965-24-1 -
 Tamoxifen Citrate PowderView Details
Tamoxifen Citrate PowderView Details
10540-29-1 -
 Tamoxifen Citrate Api, Grade Standard: IPView Details
Tamoxifen Citrate Api, Grade Standard: IPView Details
54965-24-1 -
 C32H37NO8 Tamoxifen Citrate Cas 54965 24 1, 20mgView Details
C32H37NO8 Tamoxifen Citrate Cas 54965 24 1, 20mgView Details
54965-24-1 -
 C32H37NO8 Tamoxifen Citrate PowderView Details
C32H37NO8 Tamoxifen Citrate PowderView Details
54965-24-1 -
 Tamoxifen Citrate USPView Details
Tamoxifen Citrate USPView Details
54965-24-1
