Mifepristone
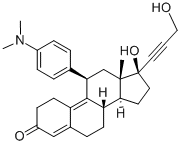
Synonym(s):(11β,17β)-11-(4-(Dimethylamino)phenyl)-17-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one, RU-486;11β-(4-Dimethyl-amino)-phenyl-17β-hydroxy-17-(1-propynyl)-estra-4,9-dien-3-one;Mifepristone - CAS 84371-65-3 - Calbiochem;RU-38486;RU-486
- CAS NO.:84371-65-3
- Empirical Formula: C29H35NO2
- Molecular Weight: 429.59
- MDL number: MFCD00867226
- EINECS: 617-559-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
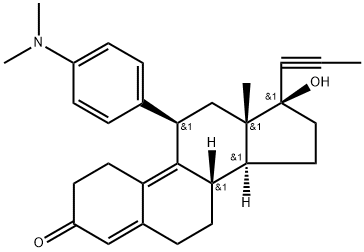
What is Mifepristone?
Absorption
The absolute bioavailability of a 20 mg oral dose is 69%
Toxicity
Nearly all of the women who receive mifepristone will report adverse reactions, and many can be expected to report more than one such reaction. About 90% of patients report adverse reactions following administration of misoprostol on day three of the treatment procedure. Side effects include more heavy bleeding than a heavy menstrual period, abdominal pain, uterine cramping, nausea, vomiting, and diarrhea.
Description
Mifepristone is an orally-active progesterone and glucocorticoid receptor antagonist indicated for use as a post-coital contraceptive. In addition to being an abortifacient, mifepristone is reported to be effective in the treatment of ocular hypertension; its potential therapeutic effect in hormone-dependent tumors is currently under investigation.
Description
Mifepristone, also known as RU-486, is a steroid derivative that was first developed as a contraceptive and for inducing abortions in pregnant women. It is quite controversial and has been banned in some countries. More?recently, mifepristone derivatives have been studied for use in diagnostic imaging and treating breast cancer.
Chemical properties
Pale Yellow Solid
Originator
Roussel-Uclaf (France)
The Uses of Mifepristone
Mifepristone is a progesterone receptor antagonist with partial agonist activity. Abortifacient.
The Uses of Mifepristone
A progesterone receptor antagonist with partial agonist activity. Abortifacient.
The Uses of Mifepristone
glutamate uptake inhibitor, AMPA blocker
The Uses of Mifepristone
A progesterone and glucocorticoid antagonist, suppresses VEGF production.
Indications
For the medical termination of intrauterine pregnancy through 49 days' pregnancy. Also indicated to control hyperglycemia secondary to hypercortisolism in adult patients with endogenous Cushing's syndrome who have type 2 diabetes mellitus or glucose intolerance and are not candidates for surgery or have had unsuccessful surgery.
Background
Mifepristone is a progestational and glucocorticoid hormone antagonist. Its inhibition of progesterone induces bleeding during the luteal phase and in early pregnancy by releasing endogenous prostaglandins from the endometrium or decidua. As a glucocorticoid receptor antagonist, the drug has been used to treat hypercortisolism in patients with nonpituitary cushing syndrome. The two marketed forms of mifepristone are Mifeprex? (mifepristone 200mg) and Korlym? (mifepristone 300mg). Currently under investigation for use in psychotic depression (phase 3 trials).
What are the applications of Application
Mifepristone is a progesterone and glucocorticoid antagonist, suppresses VEGF production
Indications
Mifepristone is a progesterone receptor antagonist that has a high affinity for glucocorticoid receptors and little agonist effect.This drug has recently been approved for use in the United States for the treatment of hypercortisolism. At high doses, mifepristone blocks negative feedback of the hypothalamic–pituitary axis, thereby increasing endogenous corticotrophin and cortisol levels. Because mifepristone exerts its effects at the receptor level and not by altering glucocorticoid production, elevated serum cortisol and corticotrophin levels may not accurately reflect the effectiveness of the therapeutic regimen. Mifepristone does not inhibit cortisol binding to the mineralocorticoid receptor, so that the resulting corticotrophin disinhibition may cause potassium depletion. Thus, administration of a mineralocorticoid receptor antagonist such as spironolactone may be indicated with mifepristone. Hypoadrenalism, nausea, and drowsiness have been reported during prolonged administration of mifepristone.
Definition
ChEBI: Mifepristone is a 3-oxo-Delta(4) steroid, an acetylenic compound and a tertiary amino compound. It has a role as an abortifacient, a contraceptive drug, a synthetic oral contraceptive and a hormone antagonist. It derives from a hydride of an estrane.
Manufacturing Process
1st method of synthesis of mifepristone:A solution of 24 g of 4-(N,N-dimethylaminoethoxy)bromobenzene was addeddropwise over 45 min to magnesium in 90 ml of anhydrous tetrahydrofuran. 2ml of 1,2-dibromoethane were added as catalyst. After the addition, themixture was stirred at 25°C for one hour to obtain a solution of 0.7 M of 4-(N,N-dimethylaminoethoxy)-benzene magnesium bromide which was thenadded to a solution of 6.16 g of dimethylsulfide-cuprous bromide complex in20 ml of tetrahydrofuran. The mixture was stirred at room temperature for 20min and a solution of 3.7 g of 3,3-[1,2-(ethanediyl-bisoxy)]-5α,10α-epoxy-17α-prop-1-ynyl-δ(9(11))-estrene-17β-ol in 50 ml of tetrahydrofuran was addedthereto dropwise over a few minutes. The mixture was stirred under an inertatmosphere for one hour and was then poured into a solution of 15 g ofammonium chloride in 20 ml of iced water. The mixture was extracted withether and the organic phase was washed with aqueous saturated sodiumchloride solution, was dried and evaporated to dryness under reducedpressure. The 18.3 g of oil were chromatographed over silica gel and elutedwith chloroform to obtain 4.5 g of 3,3-[1,2-ethanediyl-bisoxy]-11β-[4-(N,N-dimethylaminoethoxy)phenyl]-17α-(prop-1-ynyl)-δ9-estrene-5α,17β-diol with aspecific rotation of [α]D20=-44(+/-)1.5° (c = 1% in chloroform).
9.5 ml of 2 N hydrochloric acid were added to a solution of 4.5 g of 3,3-[1,2-ethanediyl-bisoxy]-11β-[4-(N,N-dimethylaminoethoxy)phenyl]-17α-(prop-1-ynyl)-δ9-estrene-5α,17β-diol in 20 ml of methanol and the solution was stirredat room temperature for 2 hours. 260 ml of ether and 110 ml of an aqueoussaturated sodium bicarbonate solution were added to the mixture which wasstirred at room temperature for 15 min. The decanted aqueous phase wasextracted with ether and the organic phase was dried and evaporated todryness under reduced pressure. The 3.3 g of residue were chromatographedover silica gel and eluted with a 92.5/7.5 methylene chloride-methanolmixture to obtain 1.8 g of amorphous 11β-[4-(N,N-dimethylaminoethoxy)phenyl]-17α-(prop-1-ynyl)-δ4,9-estradiene-17β-ol-3-one with a specificrotation of [α]D20=+71° (c = 1% in chloroform).
2th method of synthesis of mifepristone (see scheme):The oxidation of the diene I, which constitutes an intermediate for totalsynthesis of 19-nor steroids, with a reagent prepared from trifluoroaceticanhydride/hydrogen peroxide was obtained exclusively α-epoxide II. Thecondensation of II with the Grignard reagent from 4-bromo-N,N-dimethylaniline results in addition of the reagent at the 11β-position. Thisresults in rearragement of the olefin to 9,10 and opening of the epoxide. Thestereochemistry of the product obtained III is consistent with trans-opening ofthe oxirane, albeit at a remove of two carbon atoms. Mild hydrolysis removesthe silyl cyanohydrin protecting group at the 17-position to give a ketone IV.Reaction of the ketone with propargyl lithium leads to V. Hydrolysis of thatproduct under more strenuous condition results in removal of the acetal at 3;the resulting β-hydroxyketone then dehydrates to afford the 4,10(9)-dienoneVI. Another name of VI is estra-4,9-dien-3-one, 11-(4-(dimethylamino)phenyl)-17-hydroxy-17-(1-propynyl)-, (11β,17β)- or mifepristone.
brand name
Mifeprex (Danco);Ru-486;Mifegyne.
Therapeutic Function
Antiprogesterone
World Health Organization (WHO)
Mifepristone, an antiprogesterone used in combination with a prostaglandin for the termination of early pregnancy, was introduced in 1990. Use of the combination has been associated with episodes of coronary spasm that are attributed to administration of the prostaglandin and which have resulted in several cases of cardiac infarction and ventricular fibrillation. At least one of these incidents has been fatal.
Biological Activity
Selective antagonist at progesterone (PR) and glucocorticoid (GR) receptors in vitro and in vivo . Is a silent antagonist at PR and has a higher affinity than progesterone. Has higher affinity for GR than dexamethasone.
Biochem/physiol Actions
Therefore, mifepristone is considered to be a potent abortifacient. It is also known to inhibit human chorionic gonadotropin. Mifepristone results in decidual necrosis.
Pharmacokinetics
Mifepristone is a synthetic steroid with antiprogestational effects indicated for the medical termination of intrauterine pregnancy through 49 days' pregnancy. Doses of 1 mg/kg or greater of mifepristone have been shown to antagonize the endometrial and myometrial effects of progesterone in women. During pregnancy, the compound sensitizes the myometrium to the contraction-inducing activity of prostaglandins. Mifepristone also exhibits antiglucocorticoid and weak antiandrogenic activity. The activity of the glucocorticoid dexamethasone in rats was inhibited following doses of 10 to 25 mg/kg of mifepristone. Doses of 4.5 mg/kg or greater in human beings resulted in a compensatory elevation of adrenocorticotropic hormone (ACTH) and cortisol.
Pharmacokinetics
Following oral administration, mifepristone is rapidly absorbed, with a peak plasmaconcentration in approximately 90 minutes , an oral bioavailability of approximately 70%, and a term inal elimination half-life of 18 hours. It is 98% protein bound, primarily to album in and α1-acid glycoprotein. Mifepristone is metabolized primarily via CYP3A4 pathways involving mono- and di-N-demethylation and terminal hydroxylation of the 17-propynyl chain. The fact that approximately 83% of the drug is recovered in the feces and 9% in the urine suggests a biliary route of elimination. Mifepristone also demonstrates antiglucocorticoid activity.
Clinical Use
An antiprogestin is a substance that competes with progesterone for its receptor and, ultimately, prevents progesterone from binding to and activating its receptor. Because progesterone is integral to the continuation of an early pregnancy, it is expected that antipro-gestins will interfere with pregnancy maintenance. In 1982, the first antiproges tin, mifepristone (RU 486), was reported. Mifepristone was shown to interrupt early stages of implantation and pregnancy in humans.
Metabolism
Hepatic. Hepatic, by Cytochrome P450 3A4 isoenzyme to the N-monodemethylated metabolite (RU 42 633); RU 42 698, which results from the loss of two methyl groups from position 11 beta; and RU 42 698, which results from terminal hydroxylation of the 17–propynyl chain.
Storage
Room temperature
References
https://pubchem.ncbi.nlm.nih.gov/compound/mifepristone#section=Top
https://www.drugbank.ca/drugs/DB00834
https://www.drugs.com/cdi/mifepristone-tablets.html
Properties of Mifepristone
| Melting point: | 195-198°C |
| Boiling point: | 544.13°C (rough estimate) |
| alpha | D20 +138.5° (c = 0.5 in chloroform) |
| Density | 1.0731 (rough estimate) |
| refractive index | 1.6290 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in DMSO (up to 40 mg/ml) or in Ethanol (up to 20 mg/ml). |
| form | Yellow solid |
| pka | 12.94±0.60(Predicted) |
| color | Yellow |
| Water Solubility | 474.8ug/L(22.5 ºC) |
| Merck | 14,6186 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 84371-65-3(CAS DataBase Reference) |
Safety information for Mifepristone
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Mifepristone
| InChIKey | VKHAHZOOUSRJNA-GCNJZUOMSA-N |
Mifepristone manufacturer
Clickchem Research LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 Mifepristone 99%View Details
Mifepristone 99%View Details -
 84371-65-3 Mifepristone 98%View Details
84371-65-3 Mifepristone 98%View Details
84371-65-3 -
 Mifepristone CAS 84371-65-3View Details
Mifepristone CAS 84371-65-3View Details
84371-65-3 -
 Mifepristone 95.00% CAS 84371-65-3View Details
Mifepristone 95.00% CAS 84371-65-3View Details
84371-65-3 -
 Mifepristone 99% (HPLC) CAS 84371-65-3View Details
Mifepristone 99% (HPLC) CAS 84371-65-3View Details
84371-65-3 -
 Mifepristone CAS 84371-65-3View Details
Mifepristone CAS 84371-65-3View Details
84371-65-3 -
 Mifepristone CAS 84371-65-3View Details
Mifepristone CAS 84371-65-3View Details
84371-65-3 -
 Mifepristone CAS 84371-65-3View Details
Mifepristone CAS 84371-65-3View Details
84371-65-3
