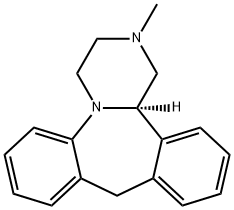
Mianserin hydrochloride
Synonym(s):1,2,3,4,10,14b-Hexahydro-2-methyl-dibenzo[c,f]pyrazino[1,2-a]azepine hydrochloride;Mianserin hydrochloride
- CAS NO.:21535-47-7
- Empirical Formula: C18H21ClN2
- Molecular Weight: 300.83
- MDL number: MFCD00055072
- EINECS: 244-426-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31

What is Mianserin hydrochloride?
Chemical properties
Off-White Solid
Originator
Tolvin,Organon,W. Germany,1975
The Uses of Mianserin hydrochloride
Mianserin hydrochloride is a tetracyclic antidepressant with an EEG and clinical activity profile similar to amitriptyline. It may cause drowsiness and hematological problems. Its mechanism of therapeutic action is not well understood, although it apparently blocks alpha-adrenergic, histamine H1, and some types of serotonin receptors.
The Uses of Mianserin hydrochloride
Mianserin hydrochloride is used as an antidepressant, antagonist, inverse agonist at 5-HT2 serotonin receptors, also blocks the H1 histamine receptor and the α2 adrenoceptor.
What are the applications of Application
Mianserin hydrochloride is a non-selective 5-HT2 receptor antagonist with moderate affinity for 5-HT6
Manufacturing Process
(A) 25 g of 2-benzylaniline dissolved in 150 ml of benzene are cooled down in
an ice bath to 8°C. To this solution are added 15 ml of pyridine and after that
a solution of 15 ml of chloroacetyl chloride in 25 ml of benzene, maintaining
the temperature of the reaction mixture at 10° to 15°C. After stirring for 1
hour at room temperature 25 ml of water are added and the mixture is
shaken for 30 minutes. Next the mixture is sucked off and the benzene layer
separated. Then the benzene layer is washed successively with 2 N HCl, a
sodium carbonate solution and water. The extract dried on sodium sulfate is
evaporated and the residue crystallized together with the crystals obtained
already from benzene. Yield 18 g; MP 130° to 133°C.
(B) 40 g of N-chloroacetyl-2-benzylaniline are heated for 2 hours at 120°C
together with 50 ml of phosphorus oxychloride and 320 g of polyphosphoric
acid. Next the reaction mixture is poured on ice and extracted with benzene.
The extract is washed and dried on sodium sulfate and the benzene distilled
off. The product obtained (31g) yields after recrystallization 24 g of 6-
chloromethyl-morphanthridine of MP 136° to 137°C.
(C) 10 g of 6-chloromethyl-morphanthridine are passed into 150 ml of a
solution of methylamine in benzene (10%). After storage of the solution for
20 hours at 0° to 5°C the methylamine hydrochloride formed is sucked off
and the filtrate evaporated to dryness. There remains as residue 11 g of crude 6-methylaminomethyl-morphanthridine.
(D) 11 g of crude 6-methylaminomethyl-morphanthridine are dissolved in 50
ml of absolute ether. While cooling in ice 2.7 g of lithium aluminumhydride,
dissolved in 100 ml of absolute ether, are added. After boiling for 1 hour and
cooling down in ice 11 ml of water are added slowly dropwise while stirring.
After stirring for another 30 minutes at room temperature the mixture is
sucked off and the filtrate evaporated to obtain 11 g of crude 5,6-dihydro-6-
methylaminomethyl-morphanthridine in the form of a light yellow oil.
(E) 10 g of 5,6-dihydro-6-methylaminomethyl-morphanthridine are heated
slowly, in 30 minutes, from 100° to 160°C with 7 g of pure diethyloxalate and
after that from 160° to 180°C in 45 minutes. After cooling down the reaction
mixture is stirred with benzene. The crystals are sucked off and yield after
crystallization from dimethylformamide 9 g of 1,2-diketo-3(N)-methyl-
2,3,4,4a-tetrahydro-1H-pyrazino-[1,2-f]-morphanthridine of MP 245° to
247°C.
(F) 9 g of the diketo-pyrazino-morphanthridine compound obtained above are
reduced with diborane to give mianserin.
Therapeutic Function
Serotonin antagonist, Antihistaminic
Biological Activity
Mianserin hydrochloride is a non-selective 5-HT2 receptor antagonist (Ki values are 0.0080 and 0.0081 μM for human 5-HT2A and 5-HT2C receptors expressed in HEK293 cells respectively). Has moderate affinity for 5-HT6. Antidepressant.
Clinical Use
Antidepressant
Drug interactions
Potentially hazardous interactions with other drugs
Alcohol: increased sedative effect.
Antibacterials: avoid with linezolid and tedizolid.
Antidepressants: avoid with MAOIs and
moclobemide.
Antiepileptics: convulsive threshold possibly
lowered; concentration reduced by carbamazepine,
fosphenytoin, phenobarbital, phenytoin and
primidone.
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Safinamide: increased risk of hypertension and CNS
excitation.
Metabolism
Mianserin is hepatically metabolised via aromatic hydroxylation, N-oxidation and N-demethylation. There are 3 urinary metabolites, (N-desmethyl, 8-hydroxy, and N-oxide derivative) which have been identified. The desmethyl metabolite, which is also active, has similar pharmacokinetic behaviour as the parent compound. Mianserin is excreted in the urine, almost entirely as its metabolites, either free or in conjugated form; 14-28% in the faeces.
Storage
Room temperature
Properties of Mianserin hydrochloride
| Melting point: | >230oC (dec.) |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: 3.4 mg/mL |
| form | neat |
| form | Solid |
| color | Off-White to Pale Yellow |
| Water Solubility | Soluble in water, ethanol, methanol, and dimethylformamide. |
| Merck | 14,6172 |
| CAS DataBase Reference | 21535-47-7(CAS DataBase Reference) |
Safety information for Mianserin hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Mianserin hydrochloride
| InChIKey | YNPFMWCWRVTGKJ-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![[2-(4-methyl-2-phenylpiperazin-1-yl)phenyl]methanol](https://img.chemicalbook.in/CAS/20200401/GIF/57321-32-1.gif)
You may like
-
 21535-47-7 Mianserin hydrochloride 98%View Details
21535-47-7 Mianserin hydrochloride 98%View Details
21535-47-7 -
 Mianserin Hydrochloride CAS 21535-47-7View Details
Mianserin Hydrochloride CAS 21535-47-7View Details
21535-47-7 -
 Mianserin HCl 98% (HPLC) CAS 21535-47-7View Details
Mianserin HCl 98% (HPLC) CAS 21535-47-7View Details
21535-47-7 -
 Mianserin hydrochloride CAS 21535-47-7View Details
Mianserin hydrochloride CAS 21535-47-7View Details
21535-47-7 -
 Mianserin hydrochloride CAS 21535-47-7View Details
Mianserin hydrochloride CAS 21535-47-7View Details
21535-47-7 -
 Mianserin hydrochloride CAS 21535-47-7View Details
Mianserin hydrochloride CAS 21535-47-7View Details
21535-47-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
