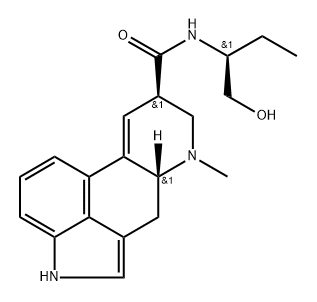
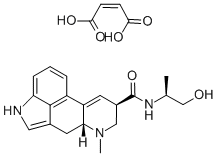
METHYSERGIDE MALEATE
Synonym(s):5-HT Serotonin Receptor Antagonsit, Methysergide Maleate, UML-491;Methysergide Maleate - CAS 129-49-7 - Calbiochem;Methysergide maleate salt
- CAS NO.:129-49-7
- Empirical Formula: C25H31N3O6
- Molecular Weight: 469.54
- MDL number: MFCD00083185
- EINECS: 204-950-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
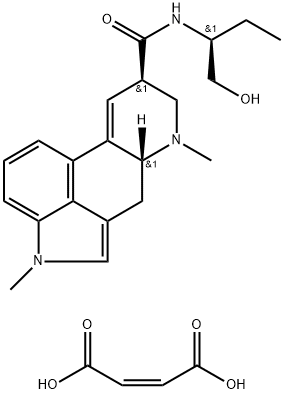
What is METHYSERGIDE MALEATE?
Description
Methysergide is an agonist of the serotonin (5-HT) receptor subtype 5-HT1 and an antagonist of 5-HT2 receptors. It binds to recombinant human 5-HT1A (KD = 23.44 nM), 5-HT1E (Ki = 229.09 nM), 5-HT1F (Ki = 33.88 nM), and rodent 5-HT1B receptors (KD = 1,513.56 nM). It also binds to recombinant human 5-HT2A (Ki = 2.69 nM) and 5-HT2C receptors (KD = 1.26 nM) and is an insurmountable antagonist at 5-HT2B receptors. It inhibits vasoconstriction induced by 5-HT in isolated postmortem human basilar arterial spiral strips (pA2 = 8.07). Methysergide decreases external carotid blood flow in a dose-dependent manner in vagosympathectomized dogs, an effect that is inhibited by the 5-HT1B/1D receptor antagonist GR127935 . It has antinociceptive activity in mouse models of pain induced by intrathecal injection of substance P , glutamate, NMDA , AMPA , or kainic acid. Methysergide reduces zymosan-induced paw edema in rats when administered at a dose of 10 mg/kg. Formulations containing methysergide were previously used in the prevention and treatment of vascular headaches.
Originator
Sansert ,Sandoz,US,1962
The Uses of METHYSERGIDE MALEATE
Methysergide maleate salt has been used as a serotonin (5HT) receptor antagonist to evaluate the potential systemic effects of methysergide (METH) and 5-HT infusions on production and milk composition in multiparous Holstein cows. It may also be used as a serotonin receptor blocker to identify the serotonergic receptor type involved in the direct excitation of mitral cells in the main olfactory bulb.
The Uses of METHYSERGIDE MALEATE
sedative
Definition
ChEBI: Methysergide maleate is an ergoline alkaloid.
Manufacturing Process
As described in US Patent 3,218,324, 0.9 part of potassium are dissolved in 500 parts by volume of liquid ammonia, then oxidized with ferric nitrate to potassium amide, after which 4.85 parts of lysergic acid-1'-hydroxybutylamide-2' are dissolved in the obtained mixture. After 15 minutes there are added to the obtained yellow solution 4.1 parts of methyl iodide in 5 parts by volume of ether, the mixture being allowed to stand for 30 more minutes at -60°C. The liquid ammonia is thereupon evaporated and the dry residue is shaken out between water and chloroform. The mixture of bases which remains after the evaporation of the chloroform is chromatographed on a column of 250 parts of aluminum oxide, the desired 1-methyl-lysergic acid-1'- hydroxy-butylamide-2' being washed into the filtrate with chloroform and chloroform-0.2% ethanol. The 1-methyl-lysergic acid-1'hydroxy-butylamide-2' crystallizes from chloroform in the form of plates which melt at 194° to 196°C. Reaction with maleic acid gives the dimaleate, melting at 187° to 188°C.
brand name
Sansert (Novartis).
Therapeutic Function
Migraine therapy
Biological Activity
Mixed 5-HT 1 /5-HT 2 receptor antagonist.
Biochem/physiol Actions
Methysergide maleate, also known as sansert, is a semisynthetic ergot alkaloid ergometrine derivative. Methysergide is a serotonin 1(5-HT1) receptor agonist and a nonselective 5-HT2 and 5-HT7 serotonin receptor antagonist. Methysergide maleate is used as a pharmacological agent to treat migraines and other vascular headaches. But, prolonged and uncontrolled use of this drug may cause Leriche′s syndrome, angina pectoris, acute ischemia of the limbs. In addition, gastrointestinal side effects, such as abdominal cramps, nausea, and diarrhea have also been observed in few patients.
Safety Profile
Poison by ingestion and intravenous routes. Experimental reproductive effects. Human mutation effects reported. When heated to decomposition it emits toxic fumes of NOx.
storage
Store at RT
Properties of METHYSERGIDE MALEATE
| storage temp. | Store at RT |
| solubility | DMSO: >10 mg/mL |
| form | solid |
| color | white to off-white |
| Water Solubility | Soluble to 10 mM in water with gentle warming |
| Stability: | Hygroscopic |
Safety information for METHYSERGIDE MALEATE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for METHYSERGIDE MALEATE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Methysergide maleate CAS 129-49-7View Details
Methysergide maleate CAS 129-49-7View Details
129-49-7 -
 Methysergide Maleate CAS 129-49-7View Details
Methysergide Maleate CAS 129-49-7View Details
129-49-7 -
 Methysergide maleate salt CAS 129-49-7View Details
Methysergide maleate salt CAS 129-49-7View Details
129-49-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4