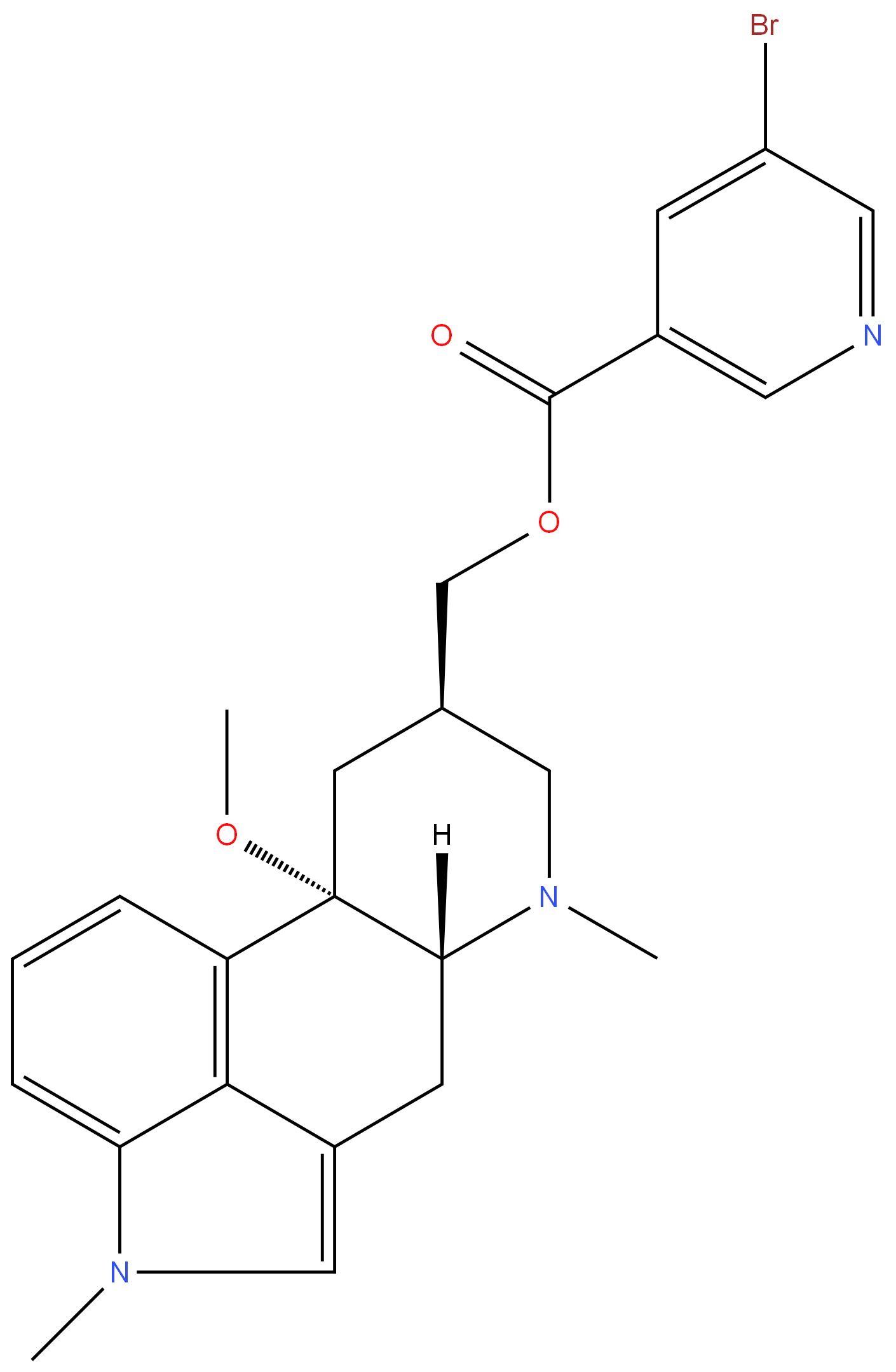
Nicergoline
Synonym(s):5-Bromonicotinic acid 10-methoxy-1,6-dimethylergoline-8-methyl ester;Nicergoline
- CAS NO.:27848-84-6
- Empirical Formula: C24H26BrN3O3
- Molecular Weight: 484.39
- MDL number: MFCD00869626
- EINECS: 248-694-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-21 17:11:28
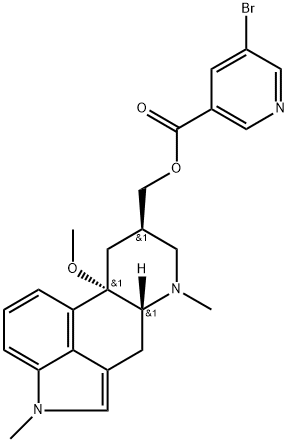
What is Nicergoline?
Chemical properties
Fine to granular, white or yellowish powder.
Originator
Sermion,Farmitalia,Italy,1974
The Uses of Nicergoline
Nicergoline is a drug used for age-dependent cognitive impairment such as Alzheimers disease and other types of dementia. Nicergoline has shown to protect cultured neurons against β-amyloid toxicity. Nicergoline protects against neuronal cell death induced by activated microglia and astrocytes through the inhibition of inflammatory mediators and the upregulation of neurotrophic factors by glial cells. Nicergoline is a drug used for age-dependent cognitive impairment and it protects cultured neurons against β-amyloid toxicity.
The Uses of Nicergoline
antipsychotic
Indications
For the treatment of senile dementia, migraines of vascular origin, transient ischemia, platelet hyper-aggregability, and macular degeneration.
Background
An ergot derivative that has been used as a cerebral vasodilator and in peripheral vascular disease. It has been suggested to ameliorate cognitive deficits in cerebrovascular disease.
What are the applications of Application
Nicergoline is an α-adrenergic modulator and vasodilator
Definition
ChEBI: Nicergoline is an organonitrogen heterocyclic compound and an organic heterotetracyclic compound.
Manufacturing Process
Preparation of 1-Methyl Lumilysergic Acid 8-Methyl Ester-10-Methyl Ether:
Into a suspension of 10 grams of 1-methyl-lumilysergic acid in 600 cc of
absolute methanol a stream of anhydrous hydrogen chloride is bubbled for 1.5
hours with strong cooling. The stream of hydrogen chloride is stopped and the
mixture is allowed to stand for 30 minutes at 0°C, and is evaporated in vacuo
to dryness. The residue is taken up with ice-cooled water made alkaline with
concentrated ammonia and extracted with chloroform. The combined
chloroform extracts are washed first with a 5% aqueous solution of sodium
bicarbonate, then with water, and are thereafter dried over anhydrous sodium
sulfate and finally evaporated in vacuo to dryness.
Preparation of 1-Methyl Lumilysergol-10-Methyl Ether: To a boiling suspension
of 2 grams of lithium aluminum hydride in 50 cc of anhydrous
tetrahydrofuran, a solution of 1 gram of 1-methyl lumilysergic acid-8-methyl
ester-10-methyl ether in 20 cc of anhydrous tetrahydrofuran is added
dropwise and the resulting solution is refluxed for a further 2 hours. After
cooling the resulting solution, aqueous tetrahydrofuran is added to destroy the
excess reducing agent and the solution is filtered. Tetrahydrofuran is distilled
off and the residue is recrystallized from acetone petroleum ether.
Preparation of Nicergoline: To a solution of 1-methyl lumilysergol-10-methyl
ether in pyridine, 5-bromonicotinyl chloride is used as an acylating agent at room temperature. The mixture is stirred for 1 hour. Water and methanol are
added and the resulting mixture is stirred for 1 hour, extracted with
chloroform, and washed in sequence with 1% aqueous caustic soda, 5%
aqueous sodium bicarbonate solution, and water. The resulting solution is
dried over anhydrous sodium sulfate and the solvent is distilled off. By
recrystallization of the residue from acetone petroleum ether, nicergoline is
obtained, melting at 136° to 138°C.
brand name
Sermion (Farmitalia, Societa Farmaceutici Italia, Italy).
Therapeutic Function
Vasodilator
Biological Activity
α -adrenergic, vasodilator. Cognitive enhancer.
Pharmacokinetics
Nicergoline is a potent vasodilator (improves brain blood flow). On the cerebral level it prompts a lowering of vascular resistance, an increase in arterial flow and stimulates the use of oxygen and glucose. Nicergoline also improves blood circulation in the lungs and limbs and has been shown to inhibit blood platelet aggregation.
Pharmacology
Nicergoline is an ergot derivative that may protect against degeneration of cholinergic neurones (Giardino et al.,2002). Nicergoline has a broad spectrum of action (Winblad et al,2008): (1) as a1-adrenoceptor antagonist it induces vasodilatation and increases arterial blood flow; (2) it enhances cholinergic and catecholaminergic neurotransmission; (3) it inhibits platelet aggregation; (4) it promotes metabolic activity, resulting in increased oxygen and glucose utilization; and (5) it has neurotrophic and antioxidant properties. Nicergoline has been used for the treatment of various dementias, including AD and VaD (Fioravanti and Flicker, 2001). The therapeutic effects of nicergoline were evident by 2 months of treatment and were maintained for 6-12 months.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion and subcutaneous routes. An experimental teratogen. Other experimental reproductive effects. A vasodilator. When heated to decomposition it emits very toxic fumes of Brand NOx.
Metabolism
Properties of Nicergoline
| Melting point: | 136-138° |
| Boiling point: | 594.4±50.0 °C(Predicted) |
| Density | 1.3558 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | Practically insoluble in water, freely soluble in methylene chloride, soluble in ethanol (96 per cent). |
| form | neat |
| pka | 6.33±0.40(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in alcohol, chloroform, and acetone. Insoluble in water. |
| Merck | 14,9496 |
Safety information for Nicergoline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Nicergoline
Abamectin manufacturer
Venkatasai Life Sciences
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 27848-84-6 Nicergoline 99%View Details
27848-84-6 Nicergoline 99%View Details
27848-84-6 -
 Nicergoline 95% CAS 27848-84-6View Details
Nicergoline 95% CAS 27848-84-6View Details
27848-84-6 -
 Nicergoline CAS 27848-84-6View Details
Nicergoline CAS 27848-84-6View Details
27848-84-6 -
 Nicergoline CAS 27848-84-6View Details
Nicergoline CAS 27848-84-6View Details
27848-84-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4