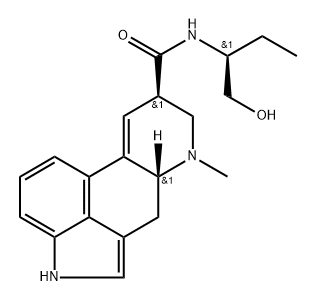
METHYLERGONOVINE
- CAS NO.:113-42-8
- Empirical Formula: C20H25N3O2
- Molecular Weight: 339.44
- MDL number: MFCD00242879
- EINECS: 204-027-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:31
What is METHYLERGONOVINE?
Absorption
Absorption is rapid after oral (60% bioavailability) and intramuscular (78% bioavailability) administration.
Toxicity
Signs and symptoms of overexposure: hypertension, seizures, headache, hypotension, nausea, and vomiting.
Chemical properties
Shiny crystals; melts at 172°C (341.6°F);moderately soluble in water, dissolves readilyin alcohol and acetone.
Originator
Methergine,Sandoz,US,1948
The Uses of METHYLERGONOVINE
Methylergonovine is a drug currently used as a smooth muscle constrictor during postpartum hemorrhage, as an inhibitor of the inflammasome complex in ASC-mediated procaspase-1 activation screening.
The Uses of METHYLERGONOVINE
Oxytocic.
The Uses of METHYLERGONOVINE
This compound is a homologue of ergonovine.It is used in the treatment of postpartumhemorrhage.
Background
A homolog of ergonovine containing one more CH2 group. (Merck Index, 11th ed)
Indications
For the prevention and control of excessive bleeding following vaginal childbirth
Definition
ChEBI: (6aR,9R)-N-[(2S)-1-hydroxybutan-2-yl]-7-methyl-6,6a,8,9-tetrahydro-4H-indolo[4,3-fg]quinoline-9-carboxamide is an ergoline alkaloid.
Manufacturing Process
To a freshly prepared solution of 2 parts of d-isolysergic acid azide in 300 parts of either is added an ethereal solution of 2 parts of d-2-aminobutanol-1 and the mixture is left to stand at room temperature during 12 hours. The yellowish clear solution is then washed several times with some water, dried over sodium sulfate and the ether evaporated in vacuo. The crystallized residue is treated with a small quantity of acetone and filtered. Yield: 2.2 parts of d-isolysergic acid-d-1-hydroxybutylamide-2. On recrystallization from
some hot methanol the new compound is obtained in form of beautiful
polygonal crystals that melt with some decomposition at 192° to 194°C
(corr.).
1 part of the iso-compound is then dissolved in 10 parts of absolute ethanol
and an alcoholic potassium hydroxide solution is added thereto. The mixture is
left to stand at room temperature during 45 minutes. After this time
equilibrium is reached between lysergic acid and the isolysergic acid forms,
which can be checked by determination of the constancy of the optical
rotation of the solution. When this point is reached, potassium hydroxide is
transformed into potassium carbonate by bubbling through the solution a
stream of carbon dioxide; the thick crystal paste of potassium carbonate is
then diluted with 50 parts of ether, filtered and washed again with 50 parts of
ether.
The alcoholic ethereal filtrate is then dried over calcined potassium carbonate
and the solution evaporated, whereby 0.9 to 1 part of a mixture of d-lysergic
acid-d-1-hydroxybutylamide-2 and of d-isolysergic acid-d-1-
hydroxybutylamide-2 is obtained. In order to separate the isomers, the
residue is dissolved in 15 parts of hot chloroform and filtered from the small
quantity of inorganic salt, whereby on cooling down, the difficultly soluble
chloroform compound of d-lysergic acid-d-1-hydroxybutylamide-2 crystallizes
out. Yield: 0.4 part. This compound can be recrystallized from hot benzene,
whereby crystals melting with some decomposition at 172°C (corr.) are
obtained. It may then be reacted with maleic acid to give the maleate.
brand name
Methergine(Novartis).
Therapeutic Function
Oxytocic
Health Hazard
The toxic effects due to methylergonovine inhumans include nausea, vomiting, dizziness,and hypertension. The oral and intravenousLD50 values in mice are 187 and 85 mg/kg,respectively.
Biological Activity
Active metabolite of methysergide ; uterotonic agent and oxytocic.
Pharmacokinetics
Methylergometrine is a semisynthetic ergot alkaloid and a derivative of ergonovine and is used for the prevention and control of postpartum and post-abortion hemorrhage. In general, the effects of all the ergot alkaloids appear to results from their actions as partial agonists or antagonists at adrenergic, dopaminergic, and tryptaminergic receptors. The spectrum of effects depends on the agent, dosage, species, tissue, and experimental or physiological conditions. All of the alkaloids of ergot significantly increase the motor activity of the uterus. After small doses contractions are increased in force or frequency, or both, but are followed by a normal degree of relaxation. As the dose is increased, contractions become more forceful and prolonged, resting tonus is markedly increased, and sustained contracture can result.
Safety Profile
Poison by ingestion and intravenous routes. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Hepatic, with extensive first-pass metabolism.
Properties of METHYLERGONOVINE
| Melting point: | 172-173℃ (decomposition) |
| Boiling point: | 475.4°C (rough estimate) |
| alpha | D20 -45° (c = 0.4 in pyridine) |
| Density | 1.1492 (rough estimate) |
| refractive index | 1.6650 (estimate) |
| storage temp. | Desiccate at RT |
| pka | pKa 6.65± 0.03(H2O,t =24,I<0.01)(Approximate) |
| color | Prisms from MeOH/Me2CO |
Safety information for METHYLERGONOVINE
Computed Descriptors for METHYLERGONOVINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1