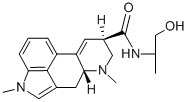
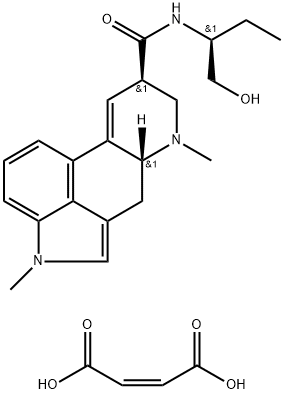
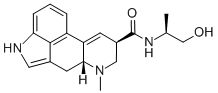
methysergide
- CAS NO.:361-37-5
- Empirical Formula: C21H27N3O2
- Molecular Weight: 353.46
- MDL number: MFCD00242883
- EINECS: 206-644-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:38
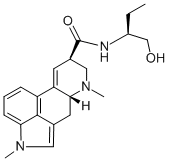
What is methysergide?
Absorption
Rapid
Toxicity
Few cases of acute methysergide intoxication have been reported. The possible symptom complex is therefore not fully known. The following symptoms are based on these few case reports. Euphoria, hyperactivity, tachycardia, dilated pupils, and dizziness have been reported in a child with a dose of 20-24 mg of methysergide. In adults, peripheral vasospasm, with diminished or absent pulses, coldness, mottling and cyanosis, has been observed at a dose of 200 mg. Ischemic tissue damage has not been reported in acute overdosage with methysergide.
Originator
Sansert,Sandoz,US,1962
The Uses of methysergide
Methysergide (Methylergonovine EP Impurity G) is a serotonergic receptor antagonist.
The Uses of methysergide
Vasoconstrictor (specific in migraine).
Indications
For the treatment of vascular headache
Background
An ergot derivative that is a congener of lysergic acid diethylamide. It antagonizes the effects of serotonin in blood vessels and gastrointestinal smooth muscle, but has few of the properties of other ergot alkaloids. Methysergide is used prophylactically in migraine and other vascular headaches and to antagonize serotonin in the carcinoid syndrome.
Definition
ChEBI: (6aR,9R)-N-[(2S)-1-hydroxybutan-2-yl]-4,7-dimethyl-6,6a,8,9-tetrahydroindolo[4,3-fg]quinoline-9-carboxamide is an ergoline alkaloid.
Manufacturing Process
As described in US Patent 3,218,324, 0.9 part of potassium are dissolved in 500 parts by volume of liquid ammonia, then oxidized with ferric nitrate to potassium amide, after which 4.85 parts of lysergic acid-1'-hydroxybutylamide-2' are dissolved in the obtained mixture. After 15 minutes there are added to the obtained yellow solution 4.1 parts of methyl iodide in 5 parts by volume of ether, the mixture being allowed to stand for 30 more minutes at -60°C. The liquid ammonia is thereupon evaporated and the dry residue is shaken out between water and chloroform. The mixture of bases which remains after the evaporation of the chloroform is chromatographed on a column of 250 parts of aluminum oxide, the desired 1-methyl-lysergic acid-1'- hydroxy-butylamide-2' being washed into the filtrate with chloroform and chloroform-0.2% ethanol. The 1-methyl-lysergic acid-1'hydroxy-butylamide-2' crystallizes from chloroform in the form of plates which melt at 194° to 196°C. Reaction with maleic acid gives the dimaleate, melting at 187° to 188°C.
Therapeutic Function
Migraine therapy
Pharmacokinetics
Methysergide has been shown, in vitro and in vivo, to inhibit or block the effects of serotonin, a substance which may be involved in the mechanism of vascular headaches. Serotonin has been variously described as a central neurohumoral agent or chemical mediator, as a "headache substance" acting directly or indirectly to lower pain threshold, as an intrinsic "motor hormone" of the gastrointestinal tract, and as a "hormone" involved in connective tissue reparative processes.
Clinical Use
Methysergide (Sansert), which is structurally identical to methylergonovine except for the addition of a methyl group to the indole nitrogen, has far less of the uterine stimulatory properties of the other agents and, instead, is used exclusively for treatment of migraine headache as a serotonin antagonist at 5-HT1/5-HT2 receptors.Methysergide is a potent peripheral inhibitor of 5-HT receptors in the blood vessel, which inhibits vessel-wall permeability. Methysergide also inhibits the release of histamine from mast cells and stabilizes platelets against the release of 5-HT, which may be responsible for the onset of the migraine attack. In contrast to its peripheral effects, methysergide appears to be a central 5-HT1 agonist, resulting in alleviation of the central hyperalgesia effect that occurs in patients with migraine.
Metabolism
Hepatic
Properties of methysergide
| Melting point: | 194-196° |
| Boiling point: | 486.87°C (rough estimate) |
| alpha | D20 -45° (c = 0.5 in pyridine) |
| Density | 1.1377 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly), Pyridine (Slightly) |
| pka | pKa 6.62± 0.02(H2O,t =24,I<0.01)(Approximate) |
| form | Solid |
| color | Off-White |
| Stability: | Light Sensitive |
Safety information for methysergide
Computed Descriptors for methysergide
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1