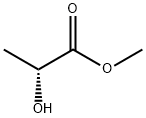
Methyl lactate
- CAS NO.:547-64-8
- Empirical Formula: C4H8O3
- Molecular Weight: 104.1
- MDL number: MFCD00066367
- EINECS: 208-930-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
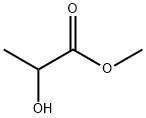
What is Methyl lactate?
Chemical properties
Methyl lactate is a colorless transparent liquid. It is soluble/miscible in water, acetone, ethyl alcohol, and other organic solvents. It is used as a cellulose acetate solvent.
The Uses of Methyl lactate
Methyl lactate is used as a solvent for nitrocellulose, cellulase acetate, cellulase acetobutyrote and cellulase acetaprapionate. It is used in the manufacture of lacquers and dopes where it contributes high tolerance for diluents, good flaw and blush resistance.
Definition
ChEBI: Methyl lactate is a lactate ester resulting from the formal condensation of the carboxy group of 2-hydroxypropanoic acid with methanol. It has a role as a metabolite. It is a lactate ester and a methyl ester.
Hazard
Moderate fire risk.
Safety Profile
Methyl lactate has been classed as non-irritant to the eyes of guinea pigs.
The estimated average lethal dose for the female rat following i.p. injection of methyl lactate was >2000 mg/kg body weight. Observations included narcosis, respiratory distress and peritoneal adhesions. The estimated non toxic dose and estimated maximum dose without gross lesions at necropsy was 500 mg/kg body weight.
Synthesis
Methyl lactate is prepared by the esterification of methyl alcohol with lactic acid.
Purification Methods
Methyl lactate is easily hydrolyzed and often contains impurities such as free lactic acid, methanol and water. Free acid can be removed by washing after neutralization with alkali. Moisture can be removed by azeotropic distillation of an azeotropic mixture with benzene. Then, it was purified by distillation under reduced pressure. Since calcium chloride and the like form molecular compounds with methyl lactate, they cannot be used as desiccants. When the methyl lactate is mixed with water, it can be extracted and recovered with benzene, ether, etc.
Properties of Methyl lactate
| Melting point: | -66°C |
| Boiling point: | 35 °C (6 mmHg) |
| alpha | [α]D20+1.410~+1.418 |
| Density | 1.093 g/mL at 20 °C(lit.) |
| refractive index | n |
| Flash point: | 49 °C |
| storage temp. | Flammables area |
| form | Liquid |
| pka | 13.07±0.20(Predicted) |
| Specific Gravity | 1.09 |
| color | Clear colorless to pale yellow |
| Water Solubility | miscible, hydrolyses |
| Sensitive | Moisture Sensitive |
| Merck | 14,6094 |
| BRN | 1720586 |
| CAS DataBase Reference | 547-64-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanoic acid, 2-hydroxy-, methyl ester(547-64-8) |
| EPA Substance Registry System | Methyl lactate (547-64-8) |
Safety information for Methyl lactate
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl lactate
| InChIKey | LPEKGGXMPWTOCB-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Methyl lactate, ≥97% CAS 547-64-8View Details
Methyl lactate, ≥97% CAS 547-64-8View Details
547-64-8 -
 Methyl Lactate CAS 547-64-8View Details
Methyl Lactate CAS 547-64-8View Details
547-64-8 -
 Methyl DL-lactate CAS 547-64-8View Details
Methyl DL-lactate CAS 547-64-8View Details
547-64-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1