Ethyl lactate
Synonym(s):Ethyl 2-hydroxypropionate
- CAS NO.:97-64-3
- Empirical Formula: C5H10O3
- Molecular Weight: 118.13
- MDL number: MFCD00065359
- EINECS: 202-598-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-21 18:03:03

What is Ethyl lactate ?
Chemical properties
Ethyl Lactate is a colorless liquid with a light ethereal, buttery odor. It miscible in water, alcohols, ketones , esters, hydrocarbons, ether and oil. The odor threshold is reported to be 0.89 mg/m3 and the odor nuisance threshold to be 65 mg/m3.
Occurrence
Ethyl lactate is reported to be found in apple, citrus fruits, pineapple, peas, sauerkraut, vinegar, bread, beer, grape brandy, rum, whisky, cider, sherry, wine, cocoa, banty beer, plum brandy and pear brandy. It has been approved by the US FDA for food use.
The Uses of Ethyl lactate
Ethyl lactate is used as a solvent for nitrocellulose, cellulose acetate, and many cellulose ethers and resins. It is also used in lacquers, paints, enamels, varnishes, stencil sheets, safety glass and flavoring. Ethyl lactate is furthermore used in some cosmetic formulations in soaps, detergents, creams and lotions with a maximum concentration of 0.2 %. In recent years it has been used as degreaser as a substitute for trichloroethylene.
Preparation
Ethyl lactate is produced by the esterification of lactic acid with ethanol in the presence of a little mineral oil, or by combination of acetaldehyde with hydrocyanic acid to form acetaldehyde cyanhydrin. This is followed by treatment with ethanol (95%) and hydrochloric or sulfuric acid. Purification is achieved using fractional distillation. The commercial product is a racemic mixture.
Definition
ChEBI: Ethyl 2-hydroxypropanoate is the ethyl ester obtained of 2-hydroxypropanoic acid. It has a role as a metabolite. It is a secondary alcohol, a lactate ester and an ethyl ester. It is functionally related to a 2-hydroxypropanoic acid.
Synthesis Reference(s)
Synthetic Communications, 19, p. 1335, 1989 DOI: 10.1080/00397918908054542
General Description
A clear colorless liquid with a mild odor. Flash point 115°F. Denser than water and soluble in water. Vapors heavier than air.
Air & Water Reactions
Flammable. Soluble in water.
Reactivity Profile
Ethyl lactate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Health Hazard
Inhalation of concentrated vapor may cause drowsiness. Contact with liquid causes mild irritation of eyes and (on prolonged contact) skin. Ingestion may cause narcosis.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety
Ethyl lactate is used as a flavoring agent in pharmaceutical
preparations, and is found in food products. The estimated
acceptable daily intake for lactic acid is 12.5 mg/kg body-weight.
In general, lactate esters have an oral LD50 > 2000 mg/kg; and
the inhalation LC50 is generally above 5000 mg/m3. They have the
potential of causing eye and skin irritation (on prolonged contact),
but not sensitization. Ethyl lactate is moderately toxic by
intraperitoneal, subcutaneous, and intravenous routes. There is
low oral and skin contact toxicity; although ingestion may cause
nausea, stomach and throat pain, and narcosis. Inhalation of
concentrated vapor of ethyl lactate may cause irritation of the
mucous membranes, drowsiness, and narcosis.
LD50 (rat, oral): >5.0 g/kg
LD50 (mouse, oral): 2.5 g/kg
LD50 (mouse, SC): 2.5 g/kg
LD50 (mouse, IV): 0.6 g/kg
LD50 (rabbit, skin): >5.0 g/kg
Veterinary Drugs and Treatments
Ethyl lactate shampoo can be used when an antibacterial shampoo (bacteriostatic and bactericidal) is needed particularly in animals with
surface and superficial pyodermas that cannot tolerate benzoyl peroxide. It also has keratoplastic effect, which provides anti-seborrheic
activity.
A lipid soluble compound, ethyl lactate penetrates hair follicles and sebaceous glands where bacterial lipases convert it into lactic acid
and ethanol, which are responsible for its antibacterial action. It is not as active as benzoyl peroxide against staphylococcal organisms, but
is less irritating and drying.
storage
Stable at normal temperature and pressure. Ethyl lactate is a flammable liquid and vapor. Store in a cool, dry, and well-ventilated location away from any fire hazard area, in a tightly closed container.
Incompatibilities
Incompatible with bases or strong alkalis and may cause fire or explosion with strong oxidizing agents.
Regulatory Status
GRAS listed. Reported in the EPA TSCA Inventory.
Properties of Ethyl lactate
| Melting point: | -26°C |
| Boiling point: | 154 °C (lit.) |
| alpha | D14 -10° |
| Density | 1.031 g/mL at 25 °C (lit.) |
| vapor pressure | 81hPa at 20℃ |
| FEMA | 2440 | ETHYL LACTATE |
| refractive index | 1.4124 |
| Flash point: | 54.6±6.4 °C |
| solubility | Miscible with water (with partial decomposition),
ethanol (95%), ether, chloroform, ketones, esters, and hydrocarbons. |
| form | clear liquid |
| pka | 13.21±0.20(Predicted) |
| color | Colorless |
| Odor | Mild characteristic. |
| optical activity | [α]20/D 10.5°, neat |
| Water Solubility | 100g/L at 20℃ |
| Merck | 14,3817 |
| JECFA Number | 931 |
| Dielectric constant | 13.1(25℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 97-64-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanoic acid, 2-hydroxy-, ethyl ester(97-64-3) |
| EPA Substance Registry System | Ethyl lactate (97-64-3) |
Safety information for Ethyl lactate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl lactate
| InChIKey | LZCLXQDLBQLTDK-UHFFFAOYSA-N |
Abamectin manufacturer
Pallav Chemicals And Solvents Pvt Ltd
New Products
4-Fluorophenylacetic acid (S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 5-Aminoimidazole-4-Carbonitrile 4-chloro-3,5-dinitropyridine 2'-Methoxy-biphenyl-2-carboxaldehyde 2-(2-Aminoethyl)isothiourea dihydrobromide, 1-(4-chlorophenyl)propan-1-one 2-Ethyl-4-methyl-1-pentanol DIISOPROPYL MALONATE PENTAFLUOROPHENOL 2-Aminonicotinic acid 6-(4-AMINOPHENYL)-5-METHYL-4,5-DIHYDRO-3(2 H)-PYRDAZINONE β-BUTYROLACTONE 3-OXO-CYCLOBUTANECARBOXYLIC ACID 3-methyl xanthine 1H-Pyrazole-3-carboxylic acid [1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3- (trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester 2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1- buten-1-yl]-, (3aR,4R,5R,6aS)- 2,5-Dibromopyridine Dimethyl (2-oxo-4-phenylbutyl)phosphonate S-(2-Chloro-3-nitrophenyl) O-ethyl carbonodithioate 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carboxylic acid 2,4-Dichloro-1-[2-nitro-4-(trifluoromethyl)phenoxy]benzeneRelated products of tetrahydrofuran


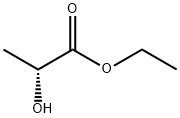

You may like
-
 97-64-3 ETHYL LACTATE 99%View Details
97-64-3 ETHYL LACTATE 99%View Details
97-64-3 -
 97-64-3 99%View Details
97-64-3 99%View Details
97-64-3 -
 Ethyl lactate 97% CAS 97-64-3View Details
Ethyl lactate 97% CAS 97-64-3View Details
97-64-3 -
 Ethyl lactate CAS 97-64-3View Details
Ethyl lactate CAS 97-64-3View Details
97-64-3 -
 Ethyl lactate CAS 97-64-3View Details
Ethyl lactate CAS 97-64-3View Details
97-64-3 -
 Ethyl Lactate CAS 97-64-3View Details
Ethyl Lactate CAS 97-64-3View Details
97-64-3 -
 Ethyl lactate CAS 97-64-3View Details
Ethyl lactate CAS 97-64-3View Details
97-64-3 -
 Ethyl lactate CAS 97-64-3View Details
Ethyl lactate CAS 97-64-3View Details
97-64-3