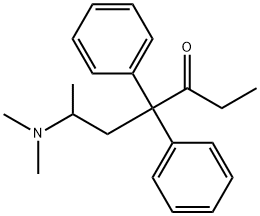
Methadone hydrochloride
Synonym(s):6-Dimethylamino-4,4-diphenylheptan-3-one hydrochloride
- CAS NO.:1095-90-5
- Empirical Formula: CH3ClO
- Molecular Weight: 66.49
- MDL number: MFCD00058014
- EINECS: 214-140-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:03:05
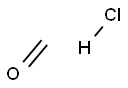
What is Methadone hydrochloride?
Description
(±)-Methadone (hydrochloride) (Item No. ISO00145) is an analytical reference material categorized as an opioid. (±)-Methadone has analgesic activity. Formulations containing (±)-methadone have been used in the treatment of opioid addiction. (±)-Methadone is regulated as a Schedule II compound in the United States. This product is intended for research and forensic applications.
Chemical properties
White Solid
Originator
Dolophine ,Lilly,US,1947
The Uses of Methadone hydrochloride
Controlled substance (opiate). Methadone hydrochloride is used in treatment of opioid dependence.
Definition
A synthetic narcotic.
Manufacturing Process
Diphenylacetonitrile is condensed with 2-chloro-1-dimethylaminopropane to give 4-(dimethylamino)-2,2-diphenyl valeronitrile. It is then reacted with ethyl magnesium bromide and then hydrolyzed using HCl to give methadone hydrochloride.
brand name
Dolophine Hydrochloride (Roxane); Dolophine Hydrochloride (Xanodyne); Methadose (Mallinckrodt); Westadone (Sandoz).
Therapeutic Function
Narcotic analgesic
Hazard
Toxic. Addictive narcotic. Use restricted.
Clinical Use
Treatment of opioid drug addiction
Analgesic for moderate to severe pain
Drug interactions
Metabolised in the liver to the Potentially hazardous interactions with other drugs
Analgesics: possible opioid withdrawal with
buprenorphine and pentazocine.
Antibacterials: metabolism increased by rifampicin;
increased risk of ventricular arrhythmias with
delamanid and telithromycin.
Antidepressants: concentration possibly increased
by fluoxetine, fluvoxamine, paroxetine and sertraline;
possible CNS excitation or depression with MAOIs
and moclobemide - avoid; possibly increased
sedative effects with tricyclics; concentration possibly
reduced by St John's wort.
Antiepileptics: concentration reduced by
carbamazepine, phenobarbital and phenytoin.
Antifungals: concentration increased by fluconazole,
ketoconazole, voriconazole and possibly itraconazole
- may need to reduce methadone dose with
voriconazole, avoid with ketoconazole.
Antihistamines: increased sedative effects with
sedating antihistamines.
Antimalarials: increased risk of ventricular
arrhythmias with piperaquine with artenimol -
avoid.
Antipsychotics: enhanced hypotensive and sedative
effects; increased risk of ventricular arrhythmias with
antipsychotics that prolong the QT interval - avoid
with amisulpride.
Antivirals: methadone possibly increases
concentration of zidovudine; concentration
reduced by efavirenz, fosamprenavir and ritonavir;
concentration possibly reduced by abacavir,
nevirapine and rilpivirine; concentration possibly
affected by boceprevir; concentration of didanosine
possibly reduced; increased risk of ventricular
arrhythmias with saquinavir and telaprevir - avoid
with saquinavir and use with caution with telaprevir.
Atomoxetine: increased risk of ventricular
arrhythmias.
Cytotoxics: possible increased risk of ventricular
arrhythmias with bosutinib, ceritinib, panobinostat
and vandetanib.
Dopaminergics: avoid with selegiline.
Nalmefene: avoid concomitant use.
Sodium oxybate: enhanced effect of sodium oxybate
- avoid
Metabolism
Metabolised in the liver to the major metabolite 2-ethylidine-1,5-dimethyl-3,3-diphenylpyrrolidine and the minor metabolite 2-ethyl-3,3-diphenyl-5- methylpyrrolidine, both of them inactive. Two other metabolites have also been identified. These metabolites are excreted in the faeces and urine with unchanged methadone
Purification Methods
The salt crystallises from EtOH, or EtOH/Et2O.
Properties of Methadone hydrochloride
| Melting point: | 232-2340C |
| Density | 1.0103 (rough estimate) |
| refractive index | 1.5790 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in water, freely soluble in ethanol (96 per cent) |
| form | powder |
| color | white to off-white |
| Water Solubility | 120 mg/mL |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 1095-90-5(CAS DataBase Reference) |
Safety information for Methadone hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
Computed Descriptors for Methadone hydrochloride
Methadone hydrochloride manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 2-Hexyn-1-ol 98+View Details
2-Hexyn-1-ol 98+View Details
764-60-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
