(+/-)-METHADONE
- CAS NO.:76-99-3
- Empirical Formula: C21H27NO
- Molecular Weight: 309.45
- MDL number: MFCD00056289
- EINECS: 200-996-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
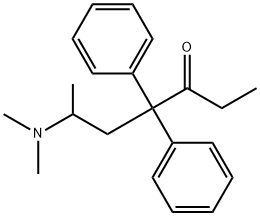
What is (+/-)-METHADONE?
Absorption
Methadone is one of the more lipid-soluble opioids and is well absorbed from the gastrointestinal tract. Following oral administration of methadone, bioavailability ranges from 36-100%, with a marked interindividual variation. It can be detected in blood as soon as 15-45 minutes following administration with peak plasma concentrations achieved between 1 to 7.5 hours. A second peak is observed ~4 hours after administration and is likely due to enterohepatic circulation. Dose proportionality of methadone pharmacokinetics is not known.
Following administration of daily oral doses ranging from 10 to 225 mg the steady-state plasma concentrations ranged between 65 to 630 ng/mL and the peak concentrations ranged between 124 to 1255 ng/mL. Effect of food on the bioavailability of methadone has not been evaluated.
Slower absorption is observed in opioid users compared to healthy subjects, which may reflect the pharmacological effect of opioids in slowing gastric emptying and mobility.
Due to the large inter-individual variation in methadone pharmacokinetics and pharmacodynamics, treatment should be individualized to each patient. There was an up to 17-fold interindividual variation found in methadone blood concentrations for a given dosage, likely due in part to individual variability in CYP enzyme function. There is also a large variability in pharmacokinetics between methadone's enantiomers, which further complicates pharmacokinetic interpretation and study.
Toxicity
In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur.
Originator
Dolophine ,Lilly,US,1947
The Uses of (+/-)-METHADONE
Analgesic (narcotic).
Indications
Methadone is indicated for the management of pain severe enough to require an opioid analgesic and for which alternative treatment options are inadequate. It's recommended that use is reserved for use in patients for whom alternative treatment options (eg, nonopioid analgesics, opioid combination products) are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient management of pain.
Methadone is also indicated for detoxification treatment of opioid addiction (heroin or other morphine-like drugs), and for maintenance substitution treatment for opioid dependence in adults in conjunction with appropriate social and medical services.
Background
Methadone is a potent synthetic analgesic that works as a full μ-opioid receptor (MOR) agonist and N-methyl-d-aspartate (NMDA) receptor antagonist. As a full MOR agonist, methadone mimics the natural effects of the body's opioids, endorphins, and enkephalins through the release of neurotransmitters involved in pain transmission. It also has a number of unique characteristics that have led to its increased use in the last two decades; in particular, methadone has a lower risk of neuropsychiatric toxicity compared to other opioids (due to a lack of active metabolites), minimal accumulation in renal failure, good bioavailability, low cost, and a long duration of action.
Due to its unique mechanism of action, methadone is particularly useful for the management of hard to treat pain syndromes such as neuropathic pain and cancer pain requiring higher and more frequent doses of shorter-acting opioids. Compared with morphine, the gold standard reference opioid, methadone also acts as an agonist of κ- and σ-opioid receptors, as an antagonist of the N-methyl-D-aspartate (NMDA) receptor, and as an inhibitor of serotonin and norepinephrine uptake. Specifically by inhibiting the NMDA receptor, methadone dampens a major excitatory pain pathway within the central nervous system. Compared to other opioids, methadone's effects on NMDA inhibition may explain it's improved analgesic efficacy and reduced opioid tolerance.
Methadone shares similar effects and risks of other opioids such as morphine, hydromorphone, oxycodone, and fentanyl. However, it also has a unique pharmacokinetic profile. Compared with short-acting and even extended-release formulations of morphine, methadone displays a comparatively longer duration of action and half-life. These effects make methadone a good option for the treatment of severe pain and addiction as fewer doses are needed to maintain analgesia and prevent opioid withdrawal symptoms. However, methadone also has an unpredictable half-life with interindividual variability, which leads to an unpredictable risk of respiratory depression and overdose when initiating or titrating therapy.
Overall, methadone's pharmacological actions result in analgesia, suppression of opioid withdrawal symptoms, sedation, miosis, sweating, hypotension, bradycardia, nausea and vomiting (via binding within the chemoreceptor trigger zone), and constipation. At higher doses, methadone use can result in respiratory depression, overdose, and death.
Treatment of opioid addiction with methadone, buprenorphine, or slow-release oral morphine (SROM) is termed Opioid Agonist Treatment (OAT) or Opioid Substitution Therapy (OST). The intention of substitution of illicit opioids with the long-acting opioids used in OAT is to prevent withdrawal symptoms for 24-36 hours following dosing to ultimately reduce cravings and drug-seeking behaviours. Use of OAT is also intended to lead to social stabilization by reducing crime rates, incarceration, use of illicit opioids such as heroin or fentanyl, and ultimately marginalization. Illegally purchased opioids present many other harms in addition to overdose as they can be injected and may be laced with other substances that increase the risk of harm or overdose. Provision of OAT is often combined with education about harm reduction including use of clean needles and injection supplies in an effort to reduce the risks associated with injection drug use such as contraction of HIV and Hepatitis C and other complications including skin infections, abscesses, or endocarditis.
Definition
ChEBI: A ketone that is heptan-3-one substituted by a dimethylamino group at position 6 and two phenyl groups at position 4.
Definition
methadone: A synthetic opioid,C21H27NO, used medically as an analgesicfor chronic pains and also as asubstitute for heroin in the treatmentof addiction. Methadone is itselfaddictive and considerablequantities of ‘street’ methadone areused in the UK.
Manufacturing Process
Diphenylacetonitrile is condensed with 2-chloro-1-dimethylaminopropane to give 4-(dimethylamino)-2,2-diphenyl valeronitrile. It is then reacted with ethyl magnesium bromide and then hydrolyzed using HCl to give methadone hydrochloride.
brand name
Dolophine Hydrochloride (Roxane); Dolophine Hydrochloride (Xanodyne); Methadose (Mallinckrodt); Westadone (Sandoz).
Therapeutic Function
Narcotic analgesic
Biological Functions
Methadone (Dolophine) has an analgesic profile and
potency similar to that of morphine but a longer duration
of action and better oral bioavailability.The kinetic
properties of methadone and its derivative, LAAM,
have been shown to be useful in the treatment of opioid
addiction.
Methadone is a useful analgesic drug for the treatment
of moderate to severe pain. Unlike morphine, it is
generally not used epidurally because of its long duration
of action. It is also rarely or never used in PCA systems
or in pregnant women during labor. The side effects
and signs of overdose following methadone
administration are similar to those observed with morphine.
Overdose is treated with naloxone. Clearance of
methadone is via the urine and bile as the cyclic Ndemethylated
drug. The ability to N-demethylate the
drug decreases in elderly patients, prolonging the action of methadone. In such patients, dosing intervals should
be longer than in younger patients. In addition, the pH
of the urine has a major effect on clearance of the drug.
Alkalinization of the urine or renal insufficiency decreases
excretion of the drug.
Drug interactions and precautions for the use of
methadone are similar to those of morphine. In addition,
rifampicin and hydantoins markedly increase the
metabolism of methadone and can precipitate withdrawal
from methadone. Conversely, the tricyclic antidepressants
and certain benzodiazepines can inhibit
metabolism of methadone, thereby increasing accumulation
of the drug, prolonging its half-life, and intensifying
its side effects. Continuous dosing with methadone
may lead to drug accumulation and to an increased incidence
of side effects; methadone is generally not used
for PCA. In pregnant heroin-addicted women, substitution
of methadone for heroin has been shown to be associated
with fewer low-birth-weight newborns and
fewer learning and cognition problems later in the life
of the child.
General Description
Methadone (Dolophine) is a synthetic opioid approved for analgesic therapy and for the maintenance and treatment of opioid addiction. Methadone is marketed as the racemate, although the opioid activity resides in the R-enantiomer (7–50 times more potent than the S-enantiomer). Methadone may only be dispensed for the treatment of opioid addiction by a program certified by the Federal Substance Abuse and Mental Health Services Administration.
Methadone is a μ-receptor agonist with complex and highly variable pharmacokinetic parameters. The major metabolic pathway of methadone metabolism is via Ndemethylation to an unstable product that spontaneously cyclizes to form the inactive 2-ethylidene-1,5-dimethyl-3,3- diphenylpyrrolidine (EDDP).
Pharmacokinetics
Overall, methadone's pharmacological actions result in analgesia, suppression of opioid withdrawal symptoms, sedation, miosis (through binding to receptors in the pupillary muscles), sweating, hypotension, bradycardia, nausea and vomiting (via binding within the chemoreceptor trigger zone), and constipation. Like many basic drugs, methadone also enters mast cells and releases histamine by a non-immunological mechanism leading to flushing, pruritus, and urticaria, which can commonly be misattributed to an allergic reaction.
Compared to other opioids, methadone has fewer active metabolites and therefore a lower risk of neuropsychiatric toxicity. This means that higher doses needed to manage severe pain or addiction are less likely to result in delirium, hyperalgesia, or seizures.
Similar to morphine, both methadone isomers are 5-HT(3) receptor antagonists, although l-methadone produces greater inhibition than d-methadone.
Methadone's effects are reversible by naloxone with a pA2 value similar to its antagonism of morphine.
Dependence and Tolerance
As with other opioids, tolerance and physical dependence may develop upon repeated administration of methadone and there is a potential for development of psychological dependence. Physical dependence and tolerance reflect the neuroadaptation of the opioid receptors to chronic exposure to an opioid and are separate and distinct from abuse and addiction. Tolerance, as well as physical dependence, may develop upon repeated administration of opioids, and are not by themselves evidence of an addictive disorder or abuse.
Patients on prolonged therapy should be tapered gradually from the drug if it is no longer required for pain control. Withdrawal symptoms may occur following abrupt discontinuation of therapy or upon administration of an opioid antagonist. Some of the symptoms that may be associated with abrupt withdrawal of an opioid analgesic include body aches, diarrhea, gooseflesh, loss of appetite, nausea, nervousness or restlessness, anxiety, runny nose, sneezing, tremors or shivering, stomach cramps, tachycardia, trouble with sleeping, unusual increase in sweating, palpitations, unexplained fever, weakness and yawning.
Cardiac Conduction Effects
Laboratory studies, both in vivo and in vitro, have demonstrated that methadone inhibits cardiac potassium channels and prolongs the QT interval. Cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. These cases appear to be more commonly associated with, but not limited to, higher dose treatment (> 200 mg/day). Methadone should be administered with particular caution to patients already at risk for development of prolonged QT interval (e.g., cardiac hypertrophy, concomitant diuretic use, hypokalemia, hypomagnesemia). Careful monitoring is recommended when using methadone in patients with a history of cardiac conduction disease, those taking medications affecting cardiac conduction, and in other cases where history or physical exam suggest an increased risk of dysrhythmia.
Respiratory Depression and Overdose
Serious, life-threatening, or fatal respiratory depression may occur with use of methadone. Patients should be
monitored for respiratory depression, especially during initiation of methadone or following a dose increase.
Respiratory depression is of particular concern in elderly or debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation. Methadone should be administered with extreme caution to patients with conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve such as: asthma, chronic obstructive pulmonary disease or cor pulmonale, severe obesity, sleep apnea syndrome, myxedema, kyphoscoliosis, and CNS depression or coma. In these patients, even usual therapeutic doses of methadone may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Alternative, non-opioid analgesics should be considered, and methadone should be employed only under careful medical supervision at the lowest effective dose.
Infants exposed in-utero or through breast milk are at risk of life-threatening respiratory depression upon delivery or when nursed.
Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, in the short-term use setting. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of opioids and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, opioids produce effects which may obscure the clinical course of patients with head injuries. In such patients, methadone must be used with caution, and only if it is deemed essential.
Incomplete Cross-tolerance between Methadone and other Opioids
Patients tolerant to other opioids may be incompletely tolerant to methadone. Incomplete cross-tolerance is of particular concern for patients tolerant to other μ-opioid agonists who are being converted to methadone, thus making the determination of dosing during opioid conversion complex. Deaths have been reported during conversion from chronic, high-dose treatment with other opioid agonists. A high degree of “opioid tolerance” does not eliminate the possibility of methadone overdose, iatrogenic or otherwise.
Crosstolerance between morphine and methadone has been demonstrated, as steady-state plasma methadone concentrations required for effectiveness (C50%) were higher in abstinent rats previously dosed with morphine, as compared to controls.
Misuse, Abuse, and Diversion of Opioids
Methadone is a mu-agonist opioid with an abuse liability similar to morphine. Methadone, like morphine and other opioids used for analgesia, has the potential for being abused and is subject to criminal diversion.
Methadone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when dispensing Methadone in situations where the clinician is concerned about an increased risk of misuse, abuse, or diversion.
Hypotensive Effect
The administration of methadone may result in severe hypotension in patients whose ability to maintain normal blood pressure is compromised (e.g., severe volume depletion).
Gastrointestinal Effects
Methadone and other morphine-like opioids have been shown to decrease bowel motility and cause constipation. This primarily occurs through agonism of opioid receptors in the gut wall. Methadone may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Sexual Function/Reproduction
Reproductive function in human males may be decreased by methadone treatment. Reductions in ejaculate volume and seminal vesicle and prostate secretions have been reported in methadone-treated individuals. In addition, reductions in serum testosterone levels and sperm motility, and abnormalities in sperm morphology have been reported. Long-term use of opioids may be associated with decreased sex hormone levels and symptoms such as low libido, erectile dysfunction, or infertility.
Pharmacology
Methadone is a diphenylpropylamine. It has very good oral bioavailability (~85%) with an oral to parenteral ratio of 1:2. Its plasma half-life can be highly variable (3–50h, average 24h) but its duration of action is relatively short. With repeated dosing, problems with accumulation can occur because of this discrepancy between half-life and analgesic effect. Careful monitoring is therefore required when converting patients to long-term methadone. A lso, there is incomplete cross-tolerance with morphine. The racemic mixture in common use has agonist actions at the MOP receptor (mainly the laevo isomer) as well as antagonist activity at the NMDA receptor dextro isomer). Given the importance of this receptor in central sensitisation in a variety of pain states, there may be cases where methadone offers particular advantages over and above other opioids (e.g. neuropathic pain). Plasma concentrations of methadone can be reduced by carbamazepine, and its metabolism is accelerated by phenytoin.
Safety Profile
Poison by ingestion, intraperitoneal, intravenous, subcutaneous, and intraduodenal routes. Human systemic effects: coma, nausea or vomiting, respiratory changes, respiratory depression, somnolence. An experimental teratogen. Experimental reproductive effects. Caution: Abuse leads to habituation or addiction. When heated to decomposition it emits toxic fumes of NOx. See also METHADONE HYDROCHLORIDE.
Metabolism
Methadone undergoes fairly extensive first-pass metabolism. Cytochrome P450 enzymes, primarily CYP3A4, CYP2B6, and CYP2C19 and to a lesser extent CYP2C9, CYP2C8, and CYP2D6, are responsible for conversion of methadone to EDDP (2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine) and other inactive metabolites, which are excreted mainly in the urine. Methadone first undergoes N-demethylation to form a highly unstable compound that spontaneously converts to EDDP through cyclization and dehydration. EDDP is then converted to 2-ethyl5-methyl-3,3-diphenyl-1-pyrroline (EDMP). Both EDDP and EDMP are inactive.
The CYP isozymes also demonstrate different affinities for metabolizing the different methadone enantiomers: CYP2C19, CYP3A7, and CYP2C8 preferentially metabolize (R)-methadone while CYP2B6, CYP2D6, and CYP2C18 preferentially metabolize (S)-methadone. CYP3A4 does not have an enantiomer preference.
Single nucleotide polymorphisms (SNPs) within the cytochrome P450 enzymes can impact methadone pharmacokinetics and contribute to the interindividual variation in response to methadone therapy. In particular, CYP2B6 polymorphisms have been shown to impact individual response to methadone as it is the predominant determinant involved in the N-demethylation of methadone, clearance, and the metabolic ratios of [methadone]/[EDDP]. The SNPs CYP2B6*6, *9, *11, CYP2C19*2, *3, CYP3A4*1B, and CYP3A5*3 result in increased methadone plasma concentrations, decreased N-demethylation, and decreased methadone clearance, while homozygous carriers of CYP2B6*6/*6 demonstrate diminished metabolism and clearance of methadone. See the pharmacogenomics section for further information.
Pharmacogenomic effects on the CYP enzymes can be significant as the long half-life of methadone can result in some individuals having higher than normal therapeutic levels which puts them at risk of dose-related side effects. For example, elevated (R)-methadone levels can increase the risk of respiratory depression, while elevated (S)-methadone levels can increase the risk of severe cardiac arrhythmias due to prolonged QTc interval.
Properties of (+/-)-METHADONE
| Melting point: | 78° |
| Boiling point: | 449.71°C (rough estimate) |
| Density | 1.0350 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| Flash point: | 9℃ |
| storage temp. | −20°C |
| pka | pKa 8.25 (Uncertain) |
| EPA Substance Registry System | 3-Heptanone, 6-(dimethylamino)-4,4-diphenyl- (76-99-3) |
Safety information for (+/-)-METHADONE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for (+/-)-METHADONE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
