Meptazinol
- CAS NO.:54340-58-8
- Empirical Formula: C15H23NO
- Molecular Weight: 233.35
- MDL number: MFCD00864339
- EINECS: 259-109-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:39
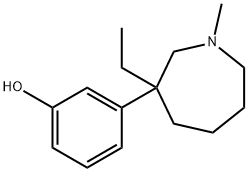
What is Meptazinol?
Originator
Meptid,Wyeth,UK,1983
The Uses of Meptazinol
Analgesic.
Definition
ChEBI: 3-(3-ethyl-1-methyl-3-azepanyl)phenol is a member of azepanes.
Manufacturing Process
2-(m-Methoxyphenyl)butyronitrile in dry ether was added to a stirred suspension of sodium amide in liquid ammonia. The mixture was stirred for 30 minutes then ethyl-4-iodobutyrate (99.25 g, 0.4 mol) in dry ether (200 ml) was added dropwise. The mixture was stirred at the temperature of refluxing liquid ammonia for 5 hours. Ammonium chloride (10 g) was added and the mixture allowed to warm to room temperature. Water (300 ml) was added, the organic layer separated, washed with water, 2 N sulfuric acid and water. After drying over magnesium sulfate and removing the ether, the product was distilled yielding ethyl 5-cyano-5-(mmethoxyphenyl)heptanoate.
That material was hydrogenated in cyclohexane using a Raney nickel catalyst. The product after distillation was recrystallized from ethyl acetate affording 10.0 g of 6-ethyl-(m-methoxyphenyl)hexahydro-2H-azepin-2one, MP 87°C to 88°C.
The azepinone (9.1 g) in dry tetrahydrofuran (50 ml) and ether (50 ml) was added dropwise to a stirred suspension of aluminum lithium hydride (7.5 g) in dry ether (50 ml). After heating under reflux for 3 hours the reaction mixture was worked up and distilled yielding 7.66 g of a compound which was a colorless oil, BP 108°C to 110°C/0.01 mm.
That product was then heated under reflux with 50% hydrobromic acid for 1.5
hours. The reaction mixture was evaporated to dryness and reevaporated with
three portions of propan-2-ol. The oil obtained was dissolved in propan-2-ol
and diluted with ether. 3-Ethyl-3-(m-hydroxyphenyl)hexahydro-1H-azepine
was obtained. That material in turn was reductively methylated by
hydrogenation in the presence of formaldehyde in absolute ethanol solution to
give 3-ethyl-3-(m-methoxyphenyl)-1-methylhexahydro-1H-azepine.
The methoxy group was converted to a hydroxy group by refluxing with 80%
HBr giving meptazinol hydrobromide.
Therapeutic Function
Analgesic
Clinical Use
Opioid analgesic used for moderate to severe pain
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: possible CNS excitation or
depression with MAOIs - avoid; possible CNS
excitation or depression with moclobemide; possibly
increased sedative effects with tricyclics.
Antihistamines: increased sedative effects with
sedating antihistamines.
Antipsychotics: enhanced hypotensive and sedative
effects.
Dopaminergics: avoid with selegiline.
Nalmefene: avoid concomitant use.
Sodium oxybate: enhanced effect of sodium oxybate
- avoid
Metabolism
Meptazinol is extensively metabolised in the liver and is excreted mainly in the urine as the glucuronide conjugate. Less than 10% of a dose has been recovered from the faeces.
Properties of Meptazinol
| Melting point: | 127-133°C |
| Boiling point: | 354.8±35.0 °C(Predicted) |
| Density | 0.997 |
| pka | 9.95±0.10(Predicted) |
Safety information for Meptazinol
Computed Descriptors for Meptazinol
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4