MAGNESIUM SILICIDE
Synonym(s):Dimagnesium silicide
- CAS NO.:22831-39-6
- Empirical Formula: Mg2Si
- Molecular Weight: 76.7
- MDL number: MFCD00016202
- EINECS: 245-254-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
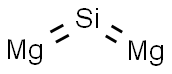
What is MAGNESIUM SILICIDE?
Description
Magnesium silicide has the molecular formula of
Mg2Si and the molecular weight of 76.6955 g/mol. Its density is 1.998 g/cc
and its melting point is 1102°C. It is a black powder.
Mg2Si crystallizes in a cubic CaF2 (fluorspar)-type
lattice with a = 4.49 ? . The centers of this lattice are analogous
to that of diamond. Each Si atom forms four covalent
sp3 bonds. The Mg subshells are only half-filled.
There is no bonding between the two Mg atom sites.
The distance between the Mg and Si atoms is 2.77 ? .
Bonding in this compound is essentially covalent.
Chemical properties
blue crystals
Chemical properties
Magnesium silicide is a slate blue crystalline solid.
Physical properties
Magnesium silicide is unstable in air and reacts with moisture to form silane. When magnesium silicide is placed into aqueous HCl, the gas silane, SiH4, is produced. This gas is the silicon analogue of methane, CH4, but is more reactive. Silane is pyrophoric, that is due to the presence of O2 in the air, i.e. it spontaneously combusts in air. These reactions are typical of a Group-2 silicide. Mg2Si reacts similarly with sulfuric acid. As a powder, magnesium silicide is dark blue or slightly purple in color.
The Uses of MAGNESIUM SILICIDE
Magnesium silicide is used to produce alloys such as aluminum alloys. It is also used in food, beverages, as a flavor enhancer and as an intermediate in chemical research.
The Uses of MAGNESIUM SILICIDE
In semiconductor research. Has been used to build Mg-Si rectifiers.
The Uses of MAGNESIUM SILICIDE
Magnesium silicide (Mg2Si) can be used as a thermoelectric material for the fabrication of thermoelectric (TE) devices. It can also be used as a counter electrode for lithium-ion batteries. It can generally be synthesized as a nanoparticle that can be utilized as a deoxygenation agent.
General Description
A colorless crystalline solid. Insoluble in water and denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion.
Air & Water Reactions
Contact with moisture under acidic condition generates silanes that ignite in air. Insoluble in water.
Reactivity Profile
MAGNESIUM SILICIDE is a reducing agent. May react vigorously with oxidizing materials.
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Potential Exposure
Magnesium silicide is used in the semiconductor industry and to produce certain aluminum alloys
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Flammable solid. Color Code—Red: FlammabilityHazard: Store in a flammable materials storage area. Priorto working with this chemical you should be trained on itsproper handling and storage. Store in tightly closed containers in a cool, well-ventilated area away from acids. Keepdry at all times. Sources of ignition, such as smoking andopen flames, are prohibited where magnesium silicide ishandled, used, or stored.
Shipping
UN2624 Magnesium silicide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet materia
Structure and conformation
The space lattice of Mg2Si belongs to the cubic system, and it has an antifluorite type structure (refer to Mg2Ge) with a lattice constant of a=0.634 nm.
Incompatibilities
Possibly pyrophoric, especially in moist air. Pyrophoric; mixtures with air are spontaneously explosive. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, mineral acids, strong acids, strong bases. Reacts with water; releasing explosive hydrogen gas and may also release selfigniting toxic silane gas
Properties of MAGNESIUM SILICIDE
| Melting point: | 1102 °C |
| Density | 1,94 g/cm3 |
| solubility | reacts with H2O |
| form | Powder |
| Specific Gravity | 2. |
| color | Blue |
| Water Solubility | Insoluble in water and denser than water. |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Crystal Structure | Cubic, Antifluorite Structure - Space Group Fm3m |
| Merck | 14,5688 |
| Stability: | Spontaneously flammable - mixtures with air are explosive. Reacts with water to form toxic vapours. |
| EPA Substance Registry System | Magnesium silicide (Mg2Si) (22831-39-6) |
Safety information for MAGNESIUM SILICIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases |
| Precautionary Statement Codes |
P231+P232:Handle under inert gas. Protect from moisture. P422:Store contents under … |
Computed Descriptors for MAGNESIUM SILICIDE
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
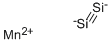
You may like
-
 Magnesium silicide CAS 22831-39-6View Details
Magnesium silicide CAS 22831-39-6View Details
22831-39-6 -
 Magnesium silicide CAS 22831-39-6View Details
Magnesium silicide CAS 22831-39-6View Details
22831-39-6 -
 Magnesium silicide CAS 22831-39-6View Details
Magnesium silicide CAS 22831-39-6View Details
22831-39-6 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
