Sodium fluorosilicate
- CAS NO.:16893-85-9
- Empirical Formula: F6Na2Si
- Molecular Weight: 188.06
- MDL number: MFCD00003491
- EINECS: 240-934-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04
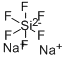
What is Sodium fluorosilicate?
Chemical properties
white powder
Chemical properties
Sodium hexafluorosilicate is a white crystalline solid.
The Uses of Sodium fluorosilicate
Sodium hexafluorosilicate is used as a additive for water fluoridation, opal glass raw material and in ore refining. It is also used in the preparation of other fluoride chemicals like sodium fluoride, sodium hexafluoroaluminate, potassium hexafluorosilicate, ammonium fluoride and calcium fluoride. It finds application as flotator, component for wet etching, wood preservative, brazing flux and welding compound. It is an active component of concrete.
The Uses of Sodium fluorosilicate
In enamels for china and porcelain; manufacture of opal glass; as insecticide, rodenticide; mothproofing of woolens. Fluoridating agent for drinking water. Intermediate in production of synthetic cryolite.
What are the applications of Application
Sodium hexafluorosilicate is a reagent for the decomposition of silicates by fusion
General Description
Fine, white, odorless, powdered solid. Toxic by ingestion, inhalation and skin absorption. Used as a rodenticide.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sodium fluorosilicate has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Hazard
Toxic by ingestion and inhalation, strong irritant to tissue.
Health Hazard
Inhalation of dust may irritate nose and throat. Ingestion causes symptoms similar to fluoride poisoning; compound is highly toxic; initial symptoms include nausea, cramps, vomiting, diarrhea, and dehydration; in severe cases, convulsions, shock, and cyanosis are followed by death in 2-4 hr. Contact with eyes causes irritation. Contact with skin causes rash, redness, and burning, sometimes followed by ulcer formation.
Fire Hazard
Behavior in Fire: Decomposes at red heat
Flammability and Explosibility
Non flammable
Industrial uses
Sodium fluorosilicate (Na2SiF6) is a white, isomorphous powder. Solubility in water is similar to that of NaF. In
acid pH, solubility improves.
Commercially, Na2SiF6 is produced by treatment of fluorosilic acid with sodium chloride,
as shown in the following reaction:
H2SiF6 + 2NaCl = Na2SiF6 + 2HCl
Safety Profile
Poison by ingestion and subcutaneous routes. A skin and severe eye irritant. An insecticide. When heated to decomposition it emits very toxic fumes of Fand Na2O.
Potential Exposure
Sodium hexafluorosilicate is as a rodenticide; as an intermediate in production of synthetic cyrolite; as an insecticide in delousing and in mothproofing of woolens.
Shipping
UN2674/Sodium fluorosilicate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise it from hot water (40mL/g) by cooling.
Incompatibilities
Reacts with acids to produce hydrogen fluoride, a highly corrosive and poisonous gas.
Properties of Sodium fluorosilicate
| Melting point: | melts at red heat, decomposing [MER06] |
| Boiling point: | 620℃[at 101 325 Pa] |
| Density | 2.68 g/mL at 25 °C(lit.) |
| vapor pressure | 0.01Pa |
| refractive index | 1.310 |
| solubility | insoluble in ethanol |
| form | Crystals |
| color | White |
| Specific Gravity | 2.68 |
| Odor | odorless |
| Water Solubility | Slightly soluble in water. Solubility increases with temperature. Insoluble in alcoholSlightly soluble in water. Insoluble in alcohol. |
| Merck | 14,8624 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability: | Stable. Incompatible with oxidizing agents, water. Moisture sensitive. |
| CAS DataBase Reference | 16893-85-9(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium fluosilicate (16893-85-9) |
Safety information for Sodium fluorosilicate
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Sodium fluorosilicate
Sodium fluorosilicate manufacturer
Zama Chemical
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Sodium hexafluorosilicate 98%View Details
Sodium hexafluorosilicate 98%View Details -
 Sodium Hexafluorosilicate pure CAS 16893-85-9View Details
Sodium Hexafluorosilicate pure CAS 16893-85-9View Details
16893-85-9 -
 Sodium Silico Fluoride, 98%, 50kg HDPE BagView Details
Sodium Silico Fluoride, 98%, 50kg HDPE BagView Details
16893-85-9 -
 Sodium Silico Fluoride, 99%, 25 Kg BagView Details
Sodium Silico Fluoride, 99%, 25 Kg BagView Details
16893-85-9 -
 Sodium Silico Fluoride, 98%, 25 Kg BagView Details
Sodium Silico Fluoride, 98%, 25 Kg BagView Details
16893-85-9 -
 Sodium Silico Fluoride, 99%, 25 Kg BagView Details
Sodium Silico Fluoride, 99%, 25 Kg BagView Details
16893-85-9 -
 Sodium Silicofluoride Powder, 99%, 50 Kg BagView Details
Sodium Silicofluoride Powder, 99%, 50 Kg BagView Details
16893-85-9 -
 Sodium Silico Fluoride, PowderView Details
Sodium Silico Fluoride, PowderView Details
16893-85-9
