CALCIUM SILICIDE
- CAS NO.:12013-56-8
- Empirical Formula: CaSi2
- Molecular Weight: 96.25
- MDL number: MFCD00015977
- EINECS: 234-588-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
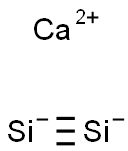
What is CALCIUM SILICIDE?
Description
Calcium silicide has the molecular formula of Ca2Si and the molecular weight of 96.2514 g/mol. This salt can be formed by the reaction of Ca metal, mixed with Si, at 850°C in an inert atmosphere.
Chemical properties
METAL PIECES
Physical properties
Calcium silicide, also called calcium disilicide, is an inorganic compound, a silicide of calcium. It is a whitish or dark gray to black solid matter with melting point between 700 and 935°C. It is insoluble in water, but may decompose when subjected to moisture, evolving hydrogen and producing calcium hydroxide. It decomposes in hot water. It is flammable and may ignite in air by producing silane that spontaneously ignites.
The Uses of CALCIUM SILICIDE
The uses of calcium silicide are many. Some of these
include:
? Calcium silicide is used for manufacture of special
metal alloys, e.g. for removing phosphorus and as
a deoxidizer.
? In pyrotechnics, it is used as fuel to make special
mixtures, e.g. for production of smokes, in flash
compositions, and in percussion caps.
? Calcium silicide is used in the manufacture of
certain initiatory, pyrotechnic and smoke
compositions.
? The silicon and calcium alloy is mainly used as
desulfurizing agent and deoxidizer for steel industry
and casting industry.
? Calcium silicide has been found to be extremely
corrosive to all metals including the refractory metals.
It has been successfully contained in boron nitride for
36 h.
? The formation of calcium silicide (CaSi2) intermetallic
through the simultaneous reduction of calcium oxide
from the electrolyte and of the silica pellet and
reduction of dissolved carbon dioxide.
C
The Uses of CALCIUM SILICIDE
It is an important raw material used in Pharmaceutical intermediates. It is an ideal compound of deoxidizer and desulfurizer which can be used in production of many types of stainless steel. It is also used as inoculants and additive in cast iron.
Properties of CALCIUM SILICIDE
| Melting point: | 1020 °C |
| Density | 2,5 g/cm3 |
| solubility | insoluble in cold H2O; reacts with hotH2O; soluble in acid solutions |
| form | pieces |
| color | Gray |
| Specific Gravity | 2.5 |
| Water Solubility | Insoluble in cold water, decomposes in hot water |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 2: reacts with aqueous acid |
| CAS DataBase Reference | 12013-56-8 |
| EPA Substance Registry System | Calcium silicide (CaSi2) (12013-56-8) |
Safety information for CALCIUM SILICIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P370+P378:In case of fire: Use … for extinction. P501:Dispose of contents/container to..… |
Computed Descriptors for CALCIUM SILICIDE
CALCIUM SILICIDE manufacturer
Bhartia Commercials Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Calcium silicide CAS 12013-56-8View Details
Calcium silicide CAS 12013-56-8View Details
12013-56-8 -
 Calcium silicide CAS 12013-56-8View Details
Calcium silicide CAS 12013-56-8View Details
12013-56-8 -
 CALCIUM SILICIDE 99%View Details
CALCIUM SILICIDE 99%View Details
12013-56-8 -
 Calcium silicide CAS 12013-56-8View Details
Calcium silicide CAS 12013-56-8View Details
12013-56-8 -
 solid Calcium Silicide, for Industrial, Packaging Size: 1 MtView Details
solid Calcium Silicide, for Industrial, Packaging Size: 1 MtView Details
12013-56-8 -
 Calcium Silicide PowderView Details
Calcium Silicide PowderView Details
12013-56-8 -
 solid Calcium Silicide, for Industrial, Packaging Size: In BagsView Details
solid Calcium Silicide, for Industrial, Packaging Size: In BagsView Details
12013-56-8 -
 Fe. Calcium SilicideView Details
Fe. Calcium SilicideView Details
12013-56-8
