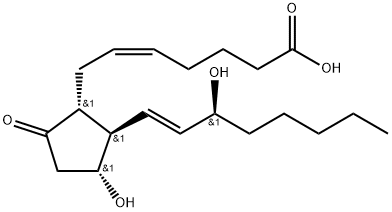
lubiprostone
- CAS NO.:333963-40-9
- Empirical Formula: C20H32F2O5
- Molecular Weight: 390.46
- MDL number: MFCD08444045
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-09-19 18:11:18
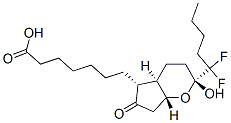
What is lubiprostone?
Description
Chronic constipation is an affliction affecting 4–5 million Americans alone. When no specific cause is identified, it is classified as idiopathic. Dietary and lifestyle modifications are the first-line conventional approaches followed by the administration of laxatives. Unfortunately, chronic idiopathic constipation is frequently refractory to traditional therapy; thus, the need for novel agents exists. Lubiprostone is a bicyclic fatty acid with a novel mechanism of action. Without affecting sodium and potassium ion concentrations, lubiprostone activates intestinal chloride ion channels, thereby, increasing intestinal water secretion and intestinal fluid chloride ion concentration. In basolateral membranepermeabilized T84 gastrointestinal epithelial cells under chloride gradient conditions, lubiprostone concentration-dependently increased short-circuit current with an EC50 of approximately 20 nM.
Originator
Sucampo (US)
The Uses of lubiprostone
Lubiprostone (Ring Closed) is a bicyclic fatty acid used for the treatment of chronic constipation and constipation associated irritable bowel syndrome (IBS-C).
brand name
Amitiza
General Description
This product is marketed as Amitiza bySucampo Pharmaceuticals, Inc. and Takeda PharmaceuticalsAmerica, Inc. to relieve chronic idiopathic constipation inadults. The recommended oral dosage is 24 μg 2 times a daywith food. Precautions and side effects are similar to thosefor other prostaglandin-derived products.
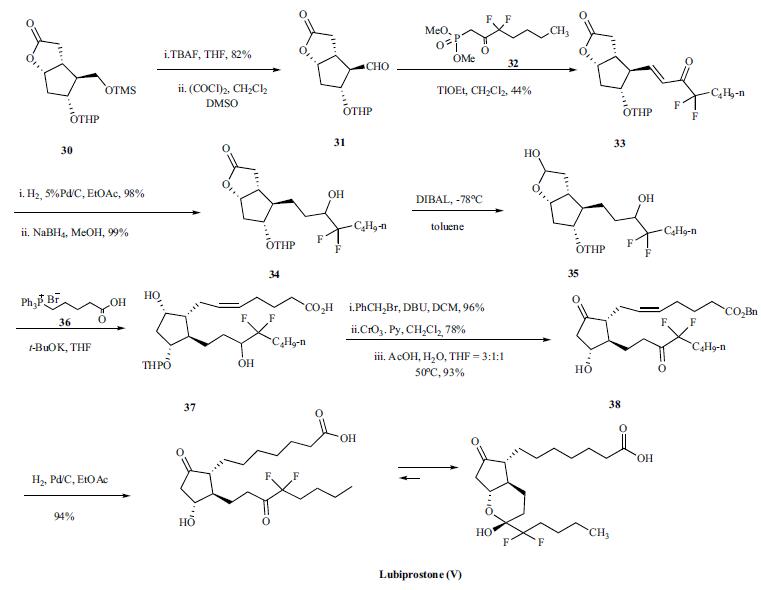
Synthesis
Synthesis of lupiprostone started with the tetrahydropyran (THP) protected (-)Corey lactone 30. Desilylation of 30 with TBAF in THF gave free carbinol in 82% yield which was oxidized with oxalyl chloride and DMSO to give corresponding crude aldehyde 31. Aldehyde 31 was condensed with dimethyl 3,3,-difluoro-2-oxoheptylphosphonate (32) in the presence of thallium ethoxide to give unsaturated difluoroketone 33 which was hydrogenated with H2 over Pd/C in ethyl acetate and the resulting ketone was subsequently reduced with sodium borohydride in methanol to give lactone 34 in excellent yield. The lactone 34 was reduced to lactol 35 with DIBAL at -78??C in toluene and the crude lactol 35 was condensed with 4-carboxybutyl triphenylphosphonium bromide (36) in the presence of t-BuOK in THF to yield compound 37. Crude 37 was reacted with benzyl bromide and DBU in dichloromethane (DCM) to give the benzyl ester in 96% yield. Oxidation of the alcohol with Collins reagent and removal of the THP protecting group under acidic conditions gave corresponding prostaglandin E2 benzyl ester 38. Finally, compound 38 was submitted to simultaneous benzyl ester group cleavage and double bond hydrogenation with H2 over Pd/C in ethyl acetate to give lubiprostone (V) in 94% yield.
Properties of lubiprostone
| Boiling point: | 532.3±50.0 °C(Predicted) |
| Density | 1.175 |
| storage temp. | -20°C Freezer |
| pka | 4.77±0.10(Predicted) |
Safety information for lubiprostone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for lubiprostone
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1