Cesium hydroxide
- CAS NO.:21351-79-1
- Empirical Formula: CsHO
- Molecular Weight: 149.91
- MDL number: MFCD00149664
- EINECS: 244-344-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
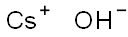
What is Cesium hydroxide?
Description
Cesium hydroxide is a colorless-to-yellowcrystalline compound. It is often used in a water solution.Molecular weight=142.92; Specific gravity (H2O:1) 53.68; Freezing/Melting point=272℃. HazardIdentification (based on NFPA 704 M Rating System):Health 3, Flammability 0, Reactivity 0. Highly soluble inwater; solubility 395% at 15℃.
Chemical properties
Cesium hydroxide is a colorless-to-yellow crystalline compound. It is often used in a water solution.
Physical properties
White to yellowish fused crystalline mass; highly deliquescent; very alkaline; density 3.68 g/cm3; melts 272°C; highly soluble in water; soluble in ethanol; aqueous solution is very alkaline.
The Uses of Cesium hydroxide
Cesium hydroxide is used in the preparation of porous cesium titanosilicates, which are potential ion-exchange materials utilized for radioactive wastes cleaning purpose. It is also involved in the preparation of other cesium salts. Further, it is used as an electrolyte in alkaline storage batteries and as a catalyst in organic synthesis. In addition to this, it is used in color photography.
What are the applications of Application
Cesium hydroxide solution is A compound used to make porous cesium titanosilicates
Preparation
Cesium hydroxide is prepared by electrolysis of cesium salts to obtain cesium metal, which then reacts with water to yield hydroxide. It also is prepared by the action of barium hydroxide with an aqueous solution of cesium sulfate.
Definition
ChEBI: Caesium hydroxide is an alkali metal hydroxide and a caesium molecular entity.
What are the applications of Application
Cesium hydroxide also exerts unusual effects in organic reactions, such as the controlled alkylation of amines. In this instance, the cesium ion itself is explicitly involved in the reaction. The hydroxide base promotes an alkylation of a primary amine, while the Cs+ ion weakly coordinates to the amine such that further alkylation is inhibited, preventing formation of dialkyl and trialkyl amines.
Cesium hydroxide solution is used in industrial catalysis. Cesium acts as apromoter e.g. in the production of polyols. Polyols are important raw materials for the production of polyurethane foams. Their synthesis profits from use of cesium hydroxide as base catalyst. In polyol synthesis isomerization of the starting material propylene oxide is an adverse side reaction eventually leading to chain termination in the polymerization with polyisocyanate. The tendency of polyols to undergo isomerization is minimized when using cesium hydroxide, as isomerization tendency decreases in the order Li > Na > K > Rb > Cs.
General Description
Cesium hydroxide is a colorless to yellow crystalline solid. Harmful to skin and eyes. Used in electric storage batteries.
Air & Water Reactions
Dilution with water may generate enough heat to cause steaming or spattering.
Reactivity Profile
CESIUM HYDROXIDE,SOLUTION neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].
Hazard
A poison. Skin, eye, and upper respiratory tract irritant.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. A powerful caustic. A corrosive skin and eye irritant. See also CESIUM.
Potential Exposure
Cesium hydroxide may be used as a raw material for other cesium salts; such as the chloride which in turn may be used to produce cesium metal. Cesium metal is used in electronic devices.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions,Cesium hydroxide 591including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. If victim is conscious, administerwater or milk. Do not induce vomiting.
Storage
Color Code—White: Corrosive or Contact Hazard:Store separately in a corrosion-resistant location. Prior toworking with cesium hydroxide you should be trained on itsproper handling and storage. Cesium hydroxide should bestored in silver or platinum away from air because it reactsviolently with oxygen. Store in tightly closed containers ina cool, well-ventilated area away from moisture and incompatible materials listed above.
Shipping
UN2682 Cesium hydroxide, Hazard class: 8; Labels: 8-Corrosive material. UN2681 Cesium hydroxide, solution, Hazard class: 8; Labels: 8-Corrosive material
Incompatibilities
Cesium hydroxide is the strongest base known. Keep away from all acids. It must be stored in silver or platinum and out of contact with air because of its reactivity with glass. CsOH causes the generation of considerable heat in contact with water or moisture. Contact with many organic compounds, many metals (i.e., aluminum, lead, tin, zinc), glass, oxygen, or carbon dioxide causes a violent reaction.
Properties of Cesium hydroxide
| Melting point: | 272 °C(lit.) |
| Density | 3.68 g/mL at 25 °C(lit.) |
| solubility | Miscible with ethanol. |
| form | Liquid |
| pka | 11.6[at 20 ℃] |
| color | Colorless |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 13,2022 |
| Exposure limits | ACGIH: TWA 2 mg/m3 NIOSH: TWA 2 mg/m3 |
| Stability: | Stable. Incompatible with acids. Absorbs carbon dioxide from the air. |
| CAS DataBase Reference | 21351-79-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Cesium hydroxide(21351-79-1) |
| EPA Substance Registry System | Cesium hydroxide (21351-79-1) |
Safety information for Cesium hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cesium hydroxide
Cesium hydroxide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

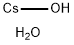
You may like
-
 Cesium hydroxide CAS 21351-79-1View Details
Cesium hydroxide CAS 21351-79-1View Details
21351-79-1 -
 Cesium hydroxide CAS 21351-79-1View Details
Cesium hydroxide CAS 21351-79-1View Details
21351-79-1 -
 Cesium hydroxide CAS 21351-79-1View Details
Cesium hydroxide CAS 21351-79-1View Details
21351-79-1 -
 Cesium hydroxide CAS 21351-79-1View Details
Cesium hydroxide CAS 21351-79-1View Details
21351-79-1 -
 Cesium hydroxide solution CAS 21351-79-1View Details
Cesium hydroxide solution CAS 21351-79-1View Details
21351-79-1 -
 Cesium hydroxide solution CAS 21351-79-1View Details
Cesium hydroxide solution CAS 21351-79-1View Details
21351-79-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
