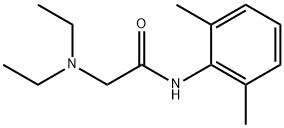
Lidocaine hydrochloride
Synonym(s):2-Diethylamino-N-(2,6-dimethylphenyl)acetamide hydrochloride monohydrate;Lignocaine hydrochloride monohydrate;Xylocaine hydrochloride monohydrate
- CAS NO.:6108-05-0
- Empirical Formula: C14H25ClN2O2
- Molecular Weight: 288.82
- MDL number: MFCD00150329
- EINECS: 612-079-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-25 18:09:02

What is Lidocaine hydrochloride?
Chemical properties
Linocaine hydrochloride is a white or almost white, crystalline powder with slightly bitter and numbing taste. Soluble in water, ethanol and organic solvents, insoluble in ether. The aqueous solution does not decompose under acid and alkali conditions, and it rarely deteriorates after repeated autoclaving.
The Uses of Lidocaine hydrochloride
Linocaine hydrochloride is a fast voltage-gated sodium channel blocker that used in Local anesthesia and heart rhythm disorders. Its actions are more intense and its effects more prolonged than those of procaine but its duration of action is shorter than that of bupivacaine or prilocaine.
What are the applications of Application
Lidocaine hydrochloride monohydrate is a fast voltage-gated sodium channel blocker
Definition
ChEBI: Linocaine hydrochloride is the monohydrate form of lidocaine hydrochloride. It is a local anesthetic and cardiac depressant used as an antiarrhythmia agent. Its actions are more intense and its effects more prolonged than those of Procaine but its duration of action is shorter than that of bupivacaine or prilocaine.
What are the applications of Application
Anesthetic (local); antiarrhythmic (class IB). Long-acting, membrane stabilizing agent against ventricular arrhythmia. Originally developed as a local anesthetic.
brand name
Alpha caine Hydrochloride (Carlisle); Anestacon (Polymedica); Laryng-O-Jet (International Medication); Lidocaton (Phar maton); Lidopen (Meridian); Xylocaine (Abraxis); Xylo caine (AstraZeneca); Xylocaine (Dentsply).
Contact allergens
Linocaine hydrochloride is an anesthetic of the amide group, like articaine or bupivacaine. Immediate-type IgE-dependent reactions are rare, and delayed-type contact dermatitis is exceptional. Cross-reactivity between the different amide anesthetics is not systematic.
Synthesis
A method for preparing Lidocaine hydrochloride is described as follows. The method comprises the following steps: by taking 2,6-xylenol as a raw material, Pd/C as a primary catalyst and 2,6-dimethylcyclohexanone as a promoter, performing liquid phase amination with ammonia water at high temperature, thereby obtaining a midbody 2,6-dimethylaniline; enabling sodium methylate, 2,6-dimethylaniline and N,N-lignocaine methyl acetate as raw materials to react at 90-95 DEGC, distilling while reaction is performed to remove methanol till no methanol can be evaporated out, continuously reacting for 30 minutes, cooling to the room temperature, adding dichloroethane, washing with water, and leaving to stand to layer, thereby obtaining an organic layer, namely, a lidocaine based dichloroethane solution; further adding hydrochloric acid into the lidocaine based dichloroethane solution, adjusting the pH value to be 3.5-4 by using hydrogen chloride, adding activated carbon to reflux for 20-40 minutes, filtering, concentrating the filtrate, cooling, crystallizing, and dying, thereby obtaining Lidocaine hydrochloride.
Mode of action
Lidocaine Hydrochloride is the hydrochloride salt form of lidocaine, an aminoethylamide and a prototypical member of the amide class anesthetics. Lidocaine interacts with voltage-gated Na+ channels in the nerve cell membrane and blocks the transient increase in permeability of excitable membranes to Na+. This prevents the generation and conduction of nerve impulses and produces a reversible loss of sensation. Lidocaine hydrochloride also exhibits class IB antiarrhythmic effects. The agent decreases the flow of sodium ions into myocardial tissue, especially on the Purkinje network, during phase 0 of the action potential, thereby decreasing depolarization, automaticity and excitability.
Properties of Lidocaine hydrochloride
| Melting point: | 75-79℃ |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | solid |
| color | white |
| Water Solubility | Soluble in water (50 mg/ml), chloroform, ethanol, and benzene. Insoluble in diethyl ether. |
| InChI | InChI=1S/C14H22N2O.ClH.H2O/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4;;/h7-9H,5-6,10H2,1-4H3,(H,15,17);1H;1H2 |
| CAS DataBase Reference | 6108-05-0(CAS DataBase Reference) |
Safety information for Lidocaine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Lidocaine hydrochloride
| InChIKey | YECIFGHRMFEPJK-UHFFFAOYSA-N |
| SMILES | C1(C(C)=CC=CC=1C)NC(=O)CN(CC)CC.O.Cl |
Lidocaine hydrochloride manufacturer
Alex Pharmachem Pvt. Ltd
Chemino Pharma Ltd
Mahendra Chemicals
Meck Pharmaceuticals and Chemicals Pvt. Ltd.
Technodrugs Intermediates Pvt. Ltd.
Ralington Pharma
Chemixl intermediates pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 6108-05-0 Lidocaine Hydrochloride 98%View Details
6108-05-0 Lidocaine Hydrochloride 98%View Details
6108-05-0 -
 Lidocaine hydrochloride 98% CAS 6108-05-0View Details
Lidocaine hydrochloride 98% CAS 6108-05-0View Details
6108-05-0 -
 Lidocaine HCl monohydrate 98% CAS 6108-05-0View Details
Lidocaine HCl monohydrate 98% CAS 6108-05-0View Details
6108-05-0 -
 Lidocaine hydrochloride CAS 6108-05-0View Details
Lidocaine hydrochloride CAS 6108-05-0View Details
6108-05-0 -
 Lidocaine hydrochloride CAS 6108-05-0View Details
Lidocaine hydrochloride CAS 6108-05-0View Details
6108-05-0 -
 Lidocaine hydrochloride CAS 6108-05-0View Details
Lidocaine hydrochloride CAS 6108-05-0View Details
6108-05-0 -
 Lidocaine hydrochloride CAS 6108-05-0View Details
Lidocaine hydrochloride CAS 6108-05-0View Details
6108-05-0 -
 Lidocaine hydrochloride monohydrate CAS 6108-05-0View Details
Lidocaine hydrochloride monohydrate CAS 6108-05-0View Details
6108-05-0