Lidocaine hydrochloride
Synonym(s):2-Diethylamino-N-(2,6-dimethylphenyl)acetamide hydrochloride monohydrate;Lidocaine hydrochloride monohydrate;Lignocaine hydrochloride monohydrate;Xylocaine hydrochloride monohydrate
- CAS NO.:73-78-9
- Empirical Formula: C14H23ClN2O
- Molecular Weight: 270.8
- MDL number: MFCD00034865
- EINECS: 200-803-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-03 14:10:04
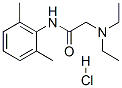
What is Lidocaine hydrochloride?
Chemical properties
White to Off-White Solid
Originator
Xylocaine,Astra,US,1949
The Uses of Lidocaine hydrochloride
Anesthetic (local); antiarrhythmic (class IB). Long-acting, membrane stabilizing agent against ventricular arrhythmia. Originally developed as a local anesthetic.
The Uses of Lidocaine hydrochloride
Local anesthesic;Na+ channel blocker
The Uses of Lidocaine hydrochloride
Apply to affected site 5 to 10 minutes before procedure. Duration of anesthesia is relatively short (<1 hour).
What are the applications of Application
Lidocaine Hydrochloride is an antiarrhythmic compound
Definition
ChEBI: Lidocaine hydrochloride is the anhydrous form of the hydrochloride salt of lidocaine. It functions as both a local anesthetic and cardiac depressant, and is commonly used as an antiarrhythmic agent. Its potency is higher and its effects last longer than those of procaine, but its duration of action is shorter than that of bupivacaine or prilocaine.
Manufacturing Process
One mol of 2,6-xylidine is dissolved in 800 ml glacial acetic acid. The mixture
is cooled to 10°C, after which 1.1 mol chloracetyl chloride is added at one
time. The mixture is stirred vigorously during a few moments after which
1,000 ml half-saturated sodium acetate solution, or other buffering or
alkalizing substance, is added at one time. The reaction mixture is shaken
during half an hour. The precipitate formed which consists of ω-chloro-2,6-
dimethyl-acetanilide is filtered off, washed with water and dried. The product
is sufficiently pure for further treatment. The yield amounts to 70 to 80% of
the theoretical amount.
One mole of the chloracetyl xylidide thus prepared and 2.5 to 3 mols diethyl
amine are dissolved in 1,000 ml dry benzene. The mixture is refluxed for 4 to
5 hours. The separated diethyl amine hydrochloride is filtered off. The benzene
solution is shaken out two times with 3N hydrochloric acid, the first time with
800 ml and the second time with 400 ml acid. To the combined acid extracts
is added an approximately 30% solution of sodium hydroxide until the
precipitate does not increase.
The precipitate, which sometimes is an oil, is taken up in ether. The ether
solution is dried with anhydrous potassium carbonate after which the ether is
driven off. The remaining crude substance is purified by vacuum distillation.
During the distillation practically the entire quantity of the substance is carried
over within a temperature interval of 1° to 2°C. The yield approaches the
theoretical amount. MP 68° to 69°C. BP 180° to 182°C at 4 mm Hg; 159° to
160°C at 2 mm Hg. (Procedure is from US Patent 2,441,498.)
Biochem/physiol Actions
Lidocaine hydrochloride is a Na+ channel blocker and an antiarrhythmic agent of the class IB, which is rapidly absorbed after parenteral administration.
brand name
Alpha caine Hydrochloride (Carlisle); Anestacon (Polymedica); Laryng-O-Jet (International Medication); Lidocaton (Phar maton); Lidopen (Meridian); Xylocaine (Abraxis); Xylo caine (AstraZeneca); Xylocaine (Dentsply).
Therapeutic Function
Local anesthetic, Antiarrhythmic
Mechanism of action
Lidocaine hydrochloride interacts with voltage-gated Na+ channels in neuronal cell membranes and blocks the transient increase in Na+ permeability of excitatory membranes. This blocks the generation and conduction of nerve impulses and produces a reversible loss of sensation. In addition, it is during phase 0 of the action potential, this agent decreases the influx of sodium ions into myocardial tissue, particularly into the Purkinje network, thereby decreasing depolarization, automaticity, and excitability.
Biological Functions
Lidocaine hydrochloride (Xylocaine) is the most commonly used local anesthetic. It is well tolerated, and in addition to its use in infiltration and regional nerve blocks, it is commonly used for spinal and topical anesthesia and as an antiarrhythmic agent. Lidocaine has a more rapidly occurring, more intense, and more prolonged duration of action than does procaine.
General Description
Lidocaine hydrochloride,2-(diethylamino)-2 ,6 -acetoxylidide monohydrochloride(Xylocaine), was conceived as a derivative of gramine(3-dimethylaminomethylindole) and introduced as a localanesthetic. It is now being used intravenously as a standardparenteral agent for suppression of arrhythmias associatedwith acute myocardial infarction and cardiac surgery.It isthe drug of choice for the parenteral treatment of prematureventricular contractions.
Mechanism of action
Lidocaine can block Na+ and K+ ion channels and regulate intracellular and extracellular calcium concentrations through other ligand-gated ion channels. Lidocaine was the first sodium channel blocker to be identified. Its main mechanism of action is blocking voltage-gated Na+ channels (VGSC/NaVs).
Clinical Use
Lidocaine hydrochloride is a class IB antiarrhythmicagent with a different effect on the electrophysiologicalproperties of myocardial cells from that of procainamideand quinidine. It binds with equal affinity to the active (A)and inactive (I) Na+ ion channels. It depresses diastolic depolarizationand automaticity in the Purkinje fiber networkand increases the functional refractory period relative toaction potential duration, as do procainamide and quinidine.It differs from the latter two drugs, however, in that it doesnot decrease, and may even enhance, conduction velocity and increase membrane responsiveness to stimulation.There are fewer data available on the subcellular mechanismsresponsible for the antiarrhythmic actions of lidocainethan on the more established drug quinidine. It has been proposedthat lidocaine has little effect on membrane cation exchangeof the atria. Sodium ion entrance into ventricularcells during excitation is not influenced by lidocaine becauseit does not alter conduction velocity in this area.Lidocaine hydrochloride does depress Na+ influx duringdiastole, as do all other antiarrhythmic drugs, to diminishautomaticity in myocardial tissue. It also alters membraneresponsiveness in Purkinje fibers, allowing increased conductionvelocity and ample membrane potential at the timeof excitation.
Safety Profile
Poison by ingestion, intraperitoneal, intravenous, subcutaneous, intramuscular, and intratracheal routes. Human systemic effects: somnolence, respiratory depression, low blood pressure, cardiomyopathy includmg infarction, pulse rate increase. An experimental teratogen. Other experimental reproductive effects. A skin and eye irritant. An anesthetic. When heated to decomposition it emits very toxic fumes of NOx and HCl.
storage
Store in a tightly closed container at room temperature away from light and moisture. Do not store in the bathroom. Do not freeze. Keep all medications away from children and pets.
Properties of Lidocaine hydrochloride
| Melting point: | 80-82°C |
| storage temp. | Inert atmosphere,2-8°C |
| form | Solid |
| color | White to off-white |
| Water Solubility | Water : ≥ 36 mg/mL (132.94 mM) |
| InChI | InChI=1S/C14H22N2O.ClH/c1-5-16(6-2)10-13(17)15-14-11(3)8-7-9-12(14)4;/h7-9H,5-6,10H2,1-4H3,(H,15,17);1H |
| CAS DataBase Reference | 73-78-9(CAS DataBase Reference) |
| EPA Substance Registry System | Acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride (73-78-9) |
Safety information for Lidocaine hydrochloride
Computed Descriptors for Lidocaine hydrochloride
| InChIKey | IYBQHJMYDGVZRY-UHFFFAOYSA-N |
| SMILES | C(NC1=C(C)C=CC=C1C)(=O)CN(CC)CC.[H]Cl |
Lidocaine hydrochloride manufacturer
Chynops Pharma
Attar Global
Pharma Links
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran


You may like
-
 29214990 98%View Details
29214990 98%View Details
29214990 -
 LIDOCAINE HYDROCHLORIDE 73-78-9 98%View Details
LIDOCAINE HYDROCHLORIDE 73-78-9 98%View Details
73-78-9 -
 Lidocaine hydrochloride 98%View Details
Lidocaine hydrochloride 98%View Details
73-78-9 -
 Lidocaine hydrochloride 98%View Details
Lidocaine hydrochloride 98%View Details
73-78-9 -
 Lidocaine HCl 73-78-9 IP/USP/BP/EPView Details
Lidocaine HCl 73-78-9 IP/USP/BP/EPView Details
73-78-9 -
 Lidocaine hydrochloride 98%View Details
Lidocaine hydrochloride 98%View Details -
 73-78-9 99%View Details
73-78-9 99%View Details
73-78-9 -
 Lidocaine hydrochloride 99%View Details
Lidocaine hydrochloride 99%View Details
73-78-9
