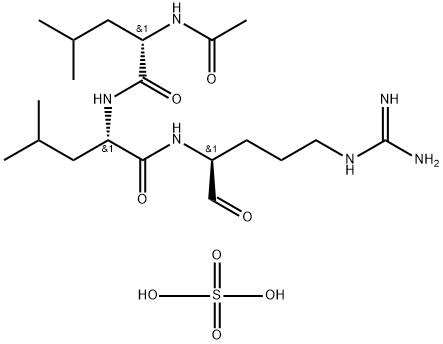
Leupeptin
Synonym(s):Leupeptin hemisulfate;Leupeptin hemisulfate salt;N-Acetyl-L -leucyl-L -leucyl-L -argininal hemisulfate salt;N-Acetyl-L -leucyl-L -leucyl-L -argininal;Acetyl-L -leucyl-L -leucylargininal
- CAS NO.:103476-89-7
- Empirical Formula: C20H40N6O8S
- Molecular Weight: 524.63
- MDL number: MFCD00081028
- EINECS: 600-443-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-18 08:51:57
What is Leupeptin?
Description
Leupeptin hemisulfate (103476-89-7) is a reversible inhibitor of trypsin-like proteases and cysteine proteases. Inhibits trypsin, plasmin, papain and cathepsin B, H and L.1-3?Blocks various apoptotic pathways in T cells.4?Commonly used in cell lysis buffers to protect proteins from degradation. Typical working concentration is 1μM (0.5 μg/ml).
Chemical properties
White to off-white lyophilized powder
The Uses of Leupeptin
Reversible protease inhibitor, which inhibits cathepsin B, calpain and trypsinPharmaceutical composition containing leupeptin hemisulfate is used as an anti-malarial agent. It is employed in the treatment of noise-induced hearing loss. It also protects the heart from myocardial stunning. Further, it is used to inhibit serine, cysteine proteases, plasmin, trypsin, papain, kallikrein and cathepsin B.
The Uses of Leupeptin
Leupeptin as well as other protease inhibitors like antipain, chymostatin, pepstatin, and phosphoramidon are useful for the protection of proteins during their isolation from tissues or membranes. Leupeptin can be removed from the reaction by dialysis.
Note: To check other protease inhibitors, try our Protease Inhibitor Set including Antipain Dihydrochloride, Aprotinin, Bestatin, Chymostatin, E-64, EDTA-Na2, Leupeptin, Pefabloc SC, Pepstatin, and Phosphoramidon.
The Uses of Leupeptin
Leupeptin has been used as a protease inhibitor: in ice-cold lysis buffer to harvest cells for western immunoblotting and immunoprecipitation,in chromatin immunoprecipitation (ChIP) lysis buffer for the isolation of fragmented chromatin samples in lysis buffer I to lyse the cells for tandem affinity purification (TAP)
What are the applications of Application
Leupeptin hemisulfate is a reversible, competitive protease inhibitor of proteases cathepsin, calpain, and trypsin
Definition
ChEBI: A peptide sulfate salt obtained by combining leupeptin with 0.5 molar equivalents of sulfuric acid.
General Description
Leupeptin, or N-acetyl-L-leucyl-L-leucyl-L-argininal, is a naturally occurring tripeptide that acts particularly as a serine protease inhibitor and as a cysteine protease inhibitor. Its mechanism of action involves structurally similar covalent binding reactions:
- In the active site of serine proteases, leupeptin forms a covalent hemiacetal adduct between the aldehyde group of leupeptin and the hydroxyl group of a serine residue in the enzyme active site.
- In the active site of cysteine proteases, the electrophilic (aldehyde) carbon of leupeptin forms a comparable bond with the sulfur atom of a cysteine residue in the enzyme active site.
Biochem/physiol Actions
Inhibitor of serine and cysteine proteases. Inhibits plasmin, trypsin, papain, calpain, and cathepsin B. Does not inhibit pepsin, cathepsins A and D, thrombin, or α-chymotrypsin. Effective concentration 10-100 μM. There have been numerous studies using leupeptin to protect against hearing loss caused by acoustic overstimulation or the ototoxic antibiotic gentamicin. (Loss of cochlear hair cells is believed to be mediated by calpain.)
storage
+4°C
References
1) Aoyagi?et al.?(1969),?Leupeptins, new protease inhibitors from Actinomycetes; J. Antibiot.,?22?283 2) Barrett?et al.?(1981),?Cathepsin B, Cathepsin H and Cathepsin L; Methods Enzymol., Pt C,?80?535 3) Knight?et al. (1980),?Human cathepsin B. Application of the substrate N-benzyloxycarbonyl-L-arginyl-L-arginine 2-naphthylamide to a study of the inhibition by leupeptin; Biochem. J.,?189?447 4) Sarin?et al. (1995),?A protease-dependent TCR-induced death pathway in mature lymphocytes; J. Immunol.,?154?5806
Properties of Leupeptin
| alpha | -76 º (c=1, water) |
| storage temp. | -20°C |
| solubility | H2O: 10 mM Solutions are stable for a week at 4 °C. Stock solutions are stable up to 6 months at −20°C. |
| form | Powder |
| color | White to Off-white |
| Water Solubility | soluble |
| Stability: | Stable for 1 year from date of purchase as supplied Solutions in distilled water, ethanol or methanol may be stored at -20°C for up to 3 months. |
Safety information for Leupeptin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P501:Dispose of contents/container to..… |
Computed Descriptors for Leupeptin
| InChIKey | CIPMKIHUGVGQTG-VFFZMTJFSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran


You may like
-
 Leupeptin hemisulfate 98%View Details
Leupeptin hemisulfate 98%View Details -
 Leupeptin, ≥95% (HPLC) CAS 103476-89-7View Details
Leupeptin, ≥95% (HPLC) CAS 103476-89-7View Details
103476-89-7 -
 Leupeptin, Hemisulfate, Microbial CAS 103476-89-7View Details
Leupeptin, Hemisulfate, Microbial CAS 103476-89-7View Details
103476-89-7 -
 Leupeptin, Hemisulfate, Synthetic CAS 103476-89-7View Details
Leupeptin, Hemisulfate, Synthetic CAS 103476-89-7View Details
103476-89-7 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0