Aprotinin
Synonym(s):Aprotinin from bovine lung;Trasylol;Trypsin inhibitor (basic);trypsin inhibitor, pancreas type (bpti);trypsin-kallikrein inhibitor
- CAS NO.:9087-70-1
- Empirical Formula: C284H432N84O78R2S7
- Molecular Weight: 6495.43988
- MDL number: MFCD00130541
- EINECS: 232-994-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-15 19:19:13
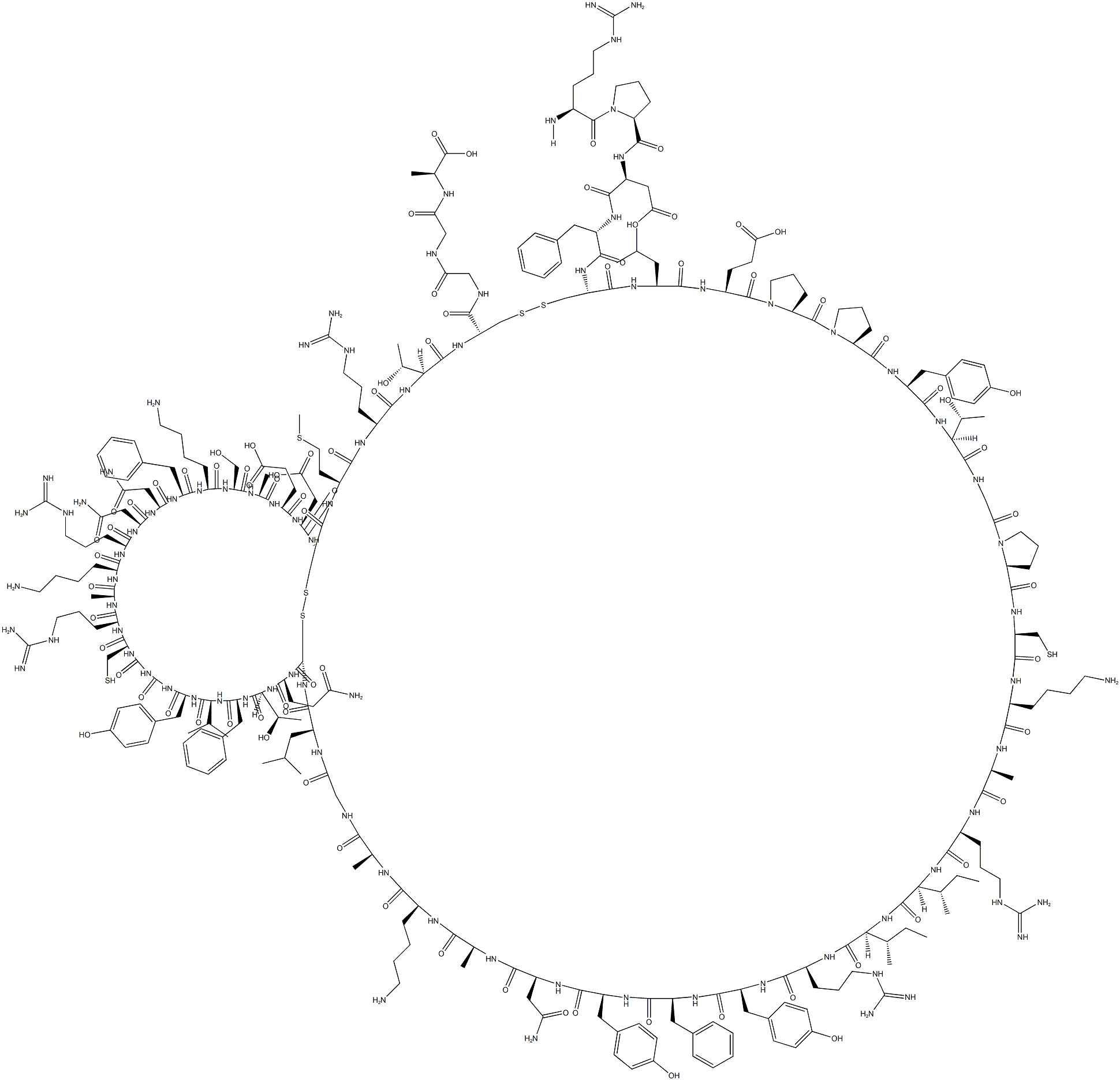
What is Aprotinin?
Description
Aprotinin is a reversible inhibitor of serine proteases such as trypsin (Ki = 0.06 pM), chymotrypsin (Ki = 9.5 nM), and kallikrein (Ki = 0.8 nM). It is a much weaker inhibitor of thrombin (Ki = 0.1-
Chemical properties
white powder
Originator
Trasylol,Bayer
The Uses of Aprotinin
Aprotinin is largely used as an inhibitor of trypsin.
The Uses of Aprotinin
A potent protease inhibitor.Aprotinin acts as a reversible serine protease blocking and a binding agent. It is widely employed as an inhibitor of trypsin. It is also used as a proteolytic inhibitor in radioimmunoassays of polypeptide hormones. It is involved in the purification of urokinase, trypsin, and chymotrypsin on immobilized aprotinin. Further, it is used in the quantification of kallikrein activity in mixtures of esterases and proteases.
What are the applications of Application
Aprotinin is a reversible serine protease blocking and binding agent which forms stable complexes and blocks active sites of serine protease enzymes
Manufacturing Process
1 kg of parotid of cattle, which were freed of fat and flesh are comminuted in
a meat chopper and twice extracted with 5 L of acetone. The acetone was
removed as far as possible by sucking off and the moist material is digested
for 2.5 hours in 1.2 L of 1 N acetic acid and 2.8 L of 96% ethanol at 50°C.
The filtrate containts about 230.000 inactivator units.
The alcoholic solution is concentrated to 1.2 L by evaporating in vacuo and
shaken with an equal amount of ether. Dark coloring matters and residues of
fat are thus dissolved. The aqueous phase is now mixed with acetone until a
precipitate occurs and the dissolved in dilute acetic acid. The solution is
brought to a weakly alkaline pH by addition of ammonia. It is then centrifuged
and the inactivator is precipitated with five times the amount of ethanol. The
yield amounts to about 180.000 kallikrein inactivator units in 0.4-0.5 g of
substance.
brand name
Trasylol (Bayer).
Therapeutic Function
Proteinase inhibitor
General Description
Trypsin inhibitor, pancreas type from bovine lung. It is known as Pancreatic trypsin inhibitor (BPTI). Aprotinin, also known as pancreatic trypsin inhibitor and trypsin-kallikrein inhibitor, is found to be expressed in lungs, spleen, liver, and pancreas. It is also found to be present in the free form in calf serum.
Biochem/physiol Actions
Aprotinin is a competitive serine protease inhibitor that forms stable complexes with and blocks the active sites of enzyme. This binding is reversible, and most aprotinin-protease complexes will dissociate at extreme pH levels >10 or <3. Structurally, Aprotinin is a monomeric globular protein derived from bovine lung that consists of 58 amino acids, arranged in a single polypeptide chain with three crosslinking disulfide bridges.
Safety Profile
Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and SOx.
storage
Store at +4°C
References
1. henry da, carless pa, moxey aj, et al. anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. cochrane database syst rev 2007; 4: cd001886.2. levi m, cromheecke me, de jonge e, et al. pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. lancet 1999; 354: 1940-7.3. sedrakyan a, treasure t, elefteriades ja. effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized
Properties of Aprotinin
| Melting point: | >234oC (dec.) |
| storage temp. | 2-8°C |
| solubility | glycerol: soluble3mg/mL, clear, colorless (equilibration buffer containing 5% glycerol) |
| form | lyophilized powder |
| color | white |
| Water Solubility | Freely soluble in water and in aqueous buffers of low ionic strength. |
| Merck | 13,761 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
Safety information for Aprotinin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Aprotinin
| InChIKey | RWLBZZCCAZDWSE-BNRYNCRGNA-N |
Aprotinin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Aprotinin CAS 9087-70-1View Details
Aprotinin CAS 9087-70-1View Details
9087-70-1 -
 Aprotinin from bovine lung CAS 9087-70-1View Details
Aprotinin from bovine lung CAS 9087-70-1View Details
9087-70-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1