Lead tetraacetate
Synonym(s):Lead tetraacetate;Pb(acac)4
- CAS NO.:546-67-8
- Empirical Formula: C8H12O8Pb-2
- Molecular Weight: 443.38
- MDL number: MFCD00008693
- EINECS: 208-908-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
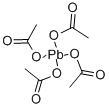
What is Lead tetraacetate?
Description
Lead (IV) acetate or lead tetraacetate is a chemical compound with chemical formula Pb(C2H3O2)4 and is a lead salt of acetic acid. It is commercially available often stabilized with acetic acid.
Chemical properties
White solid
Chemical properties
Lead tetraacetate (plumbic acetate), Pb(C2H3O2)4, is a colorless, monoclinic crystalline solid that is soluble in chloroform and in hot acetic acid, but decomposes in cold water and in ethyl alcohol. Lead tetraacetate can be prepared by adding warm, water-free, glacial acetate acid to red lead, Pb3O4, and subsequent cooling. The salt decomposes with the addition of water to give PbO2, but the yield can be improved by passing in chlorine gas. Lead tetraacetate is available in laboratory quantities as colorless to faintly pink crystals stored in glacial acetic acid.
The Uses of Lead tetraacetate
Oxidation with lead tetraacetate is often used in organic syntheses, because the lead salt is highly selective in the splitting of vicinal glycols. The rate of oxidation of cis glycols is more rapid than of the trans isomers, a property widely used in the structural determination of sugars and other polyols. Lead tetraacetate readily cleaves α-hydroxy acids as oxalic acid at room temperature. Another use is the introduction of acetoxy groups in organic molecules, as in the preparation of cyclohexyl acetate and the acetoxylation of cyclohexanol. At high temperature, methylation takes place. In these reactions, the organic molecule must contain double bonds or activating substituents.
The Uses of Lead tetraacetate
Selective oxidizing agent in organic syntheses: Criegee, Angew. Chem. 53, 321 (1940); Newer Methods of Preparative Organic Chemistry (Interscience, N. Y., 1948) pp 1-17.
The Uses of Lead tetraacetate
Lead(IV) acetate is an important oxidizing agent and a source of acetyloxy group used in organic synthesis. For example, 1,4-dioxene is prepared from dioxane involving 2-acetoxy-1,4-dioxane as an intermediate. Similarly, it is used for the preparation of bis(trifluoromethyl)diazomethane from hexafluoroacetone hydrazone. It also reacts with alkenes, alcohols having a delta-proton and di-n-butyl d-tartrate to get gamma-lactones, cyclic ethers and n-butyl glyoxylate respectively. It induces the cleavage of 1,2-diols to the corresponding aldehydes or ketones. It is actively involved in the Kochi reaction for the decarboxylation of carboxylic acids to alkyl halides and used as an alternative reagent to bromine in the Hofmann rearrangement.
What are the applications of Application
Lead(IV) acetate is induces the addition of difluorodiiodomethane to alkynes and alkenes
Definition
ChEBI: An acetate salt with formula Pb(OAc)4. It is used as a selective oxidising agent in organic synthesis.
Preparation
Lead tetraacetate can be prepared by reaction of red lead with acetic acid The other main lead acetate is lead (II) acetate.
What are the applications of Application
Lead tetraacetate is a strong oxidizing agent, a source of acetyloxy groups and a general reagent for the introduction of lead into organolead compounds. Some of its many uses in organic chemistry :
Acetoxylation of benzylic, allylic and α-oxygen ether C-H bonds, for example the photochemical conversion of dioxane to 1,4- dioxene through the 2-acetoxy-1,4-dioxane intermediate and the conversion of α-pinene to verbenone
* Oxidation of hydrazones to diazo compounds for example that of hexafluoroacetone hydrazone to bis(trifluoromethyl)diazomethane * Aziridine formation, for example the reaction of Naminophthalimide and stilbene
* Cleavage of 1,2-diols to the corresponding aldehydes or ketones often replacing ozonolysis, for instance the oxidation of di-nbutyl d-tartrate to n-butyl glyoxylate
* Reaction with alkenes to γ-lactones
* Oxidation of alcohols carrying a δ-proton to cyclic ethers.
* Oxidative cleavage of certain allyl alcohols in conjunction with ozone.
General Description
Faintly pink wet crystals with an odor of vinegar.
Air & Water Reactions
Unstable in air. Reacts with water to form brown lead dioxide and acetic acid [Merck 11th ed. 1989].
Reactivity Profile
Organometallics are strongly reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Generally highly toxic. Often react on contact with tissues to give toxic products.
Health Hazard
Early symptoms of lead intoxication by ingestion are most commonly gastrointestinal disorders, colic, constipation, etc.; weakness, which may go on to paralysis chiefly of the extensor muscles of the wrists and less often of the ankles, is noticeable in the most serious cases. Ingestion of a large amount causes local irritation of the alimentary tract; pain, leg cramps, muscle weakness, paresthesias, depression, coma, and death may follow in 1 or 2 days. Contact causes severe irritation of eyes and can burn skin.
Fire Hazard
Behavior in Fire: Can increase the intensity of a fire when in contact with combustible material. Cool containers with plenty of water.
Safety
Lead (IV) acetate may be fatal if ingested, inhaled, or absorbed through skin. It causes irritation to skin, eyes, and respiratory tract. It is a neurotoxin. It affects the gum tissue, central nervous system, kidneys, blood, and reproductive system.
Properties of Lead tetraacetate
| Melting point: | 175-180 °C |
| Boiling point: | 118.1°C |
| Density | 2,28 g/cm3 |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Chloroform, Methanol (Slightly) |
| form | Crystalline Powder |
| appearance | Colorless or pink crystals |
| color | White to light orange-pink |
| Specific Gravity | 2.28 |
| Water Solubility | Decomposition |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 7: reacts slowly with moisture/water |
| Merck | 14,5423 |
| BRN | 3595640 |
| Exposure limits | NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Stability: | Stable. Moisture sensitive. Incompatible with water, most metals. |
| CAS DataBase Reference | 546-67-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Lead tetraacetate(546-67-8) |
| EPA Substance Registry System | Lead(IV) acetate (546-67-8) |
Safety information for Lead tetraacetate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Lead tetraacetate
| InChIKey | JEHCHYAKAXDFKV-UHFFFAOYSA-J |
Lead tetraacetate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Lead Tetra Acetate 98%View Details
Lead Tetra Acetate 98%View Details -
 Lead(IV) acetate 98%View Details
Lead(IV) acetate 98%View Details
546-67-8 -
 Lead(IV) acetate 546-67-8 98%View Details
Lead(IV) acetate 546-67-8 98%View Details
546-67-8 -
 Lead(IV) acetate, 99% 546-67-8 99%View Details
Lead(IV) acetate, 99% 546-67-8 99%View Details
546-67-8 -
 Lead tetraacetate, GR CAS 546-67-8View Details
Lead tetraacetate, GR CAS 546-67-8View Details
546-67-8 -
 Lead Tetraacetate (contains Acetic Acid) CAS 546-67-8View Details
Lead Tetraacetate (contains Acetic Acid) CAS 546-67-8View Details
546-67-8 -
 Lead (IV) tetraacetate (Stabilized with 5-10% Acetic acid) 98% CAS 546-67-8View Details
Lead (IV) tetraacetate (Stabilized with 5-10% Acetic acid) 98% CAS 546-67-8View Details
546-67-8 -
 Lead Tetra AcetateView Details
Lead Tetra AcetateView Details
546-67-8
