LEAD(II) BROMIDE
Synonym(s):Lead dibromide
- CAS NO.:10031-22-8
- Empirical Formula: Br2Pb
- Molecular Weight: 367.01
- MDL number: MFCD00011156
- EINECS: 233-084-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 18:44:12
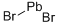
What is LEAD(II) BROMIDE?
Chemical properties
white, ortho-rhomb crystal(s); -80 mesh with 99.999% purity; enthalpy of vaporization 133 kJ/mol; enthalpy of fusion 16.44 kJ/mol; obtained from PbO or PbCO3 and HBr; finds use as a photopolymerization catalyst and in photoduplication processes in the 365 nm region [KIR78] [CER91] [CRC10] [MER06]
Physical properties
White orthorhombic crystals; density 6.66 g/cm3; melts at 373°C; forms a horn-like mass on solidification; vaporizes at 916°C; decomposes slowly on exposure to light; sparingly soluble in cold water (4.55 g/L at 0°C and 8.44 g/L at 20°C, respectively); moderately soluble in boiling water (44.1g/L at 100°C); Ksp 6.60x10-6 at 25°C; insoluble in alcohol; slightly soluble in ammonia; soluble in alkalies and also in sodium or potassium bromide solutions.
The Uses of LEAD(II) BROMIDE
It is used in the field of antirust, pigment and photograph. The molten lead(II) bromide acts as an electrolyte. Lead(II) bromide provides a high concentration of lead(II) ions and bromide ions to carry the current during the electrolysis process. The rare-earth-doped alkali-lead bromide crystals (potassium lead bromide (or) KPB; rubidium lead bromide or RPB) emerge as promising new low-phonon-energy host materials for mid-IR applications and are useful for solid state lasers. Hybrid organic/lead halide perovskites are promising materials for solar cell fabrication.
The Uses of LEAD(II) BROMIDE
Lead bromide is used for developing images in photography; as inorganic filler in fire-retardant plastics; as a photopolymerization catalyst for acrylamide monomer; and as a welding flux for welding aluminum or its alloys to other metals.
The Uses of LEAD(II) BROMIDE
Lead(II) bromide (PbBr2) can be used in the fabrication of nanoscale quasi-2D layered perovskites, which are potentially utilized as light-emitting materials. It can also be used for the synthesis of deep blue fluorescent lead bromide perovskite microdisks. These microdisks can be used as direct bandgap semiconductors for light-emitting diodes (LEDs).
Purification Methods
Crystallise it from water containing a few drops of HBr (25mL of water per gram PbBr2) between 100o and 0o. A neutral solution is evaporated at 110o, and the crystals that separate are collected by rapid filtration at 70o and dried at 105o (to give the monohydrate). Its solubility in H2O is 0.5% (at ~10o) and 5% (at ~ 100o). To prepare the anhydrous bromide, the hydrate is heated for several hours at 170o and then in a Pt boat at 200o in a stream of HBr and H2. Finally it is fused [Clayton et al. J Chem Soc, Faraday Trans 1 76 2362 1980].
Properties of LEAD(II) BROMIDE
| Melting point: | 371 °C(lit.) |
| Boiling point: | 892 °C(lit.) |
| Density | 6.66 g/mL at 25 °C(lit.) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | ethanol: insoluble(lit.) |
| form | Powder |
| color | White to off-white |
| Specific Gravity | 6.66 |
| Water Solubility | Soluble in water at 20°C 5g/L. Insoluble in ethanol. Solubility in N,N-DMF is almost transparent. |
| Merck | 14,5403 |
| Solubility Product Constant (Ksp) | pKsp: 6.82 |
| Exposure limits | ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 10031-22-8(CAS DataBase Reference) |
| EPA Substance Registry System | Lead(II) bromide (10031-22-8) |
Safety information for LEAD(II) BROMIDE
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for LEAD(II) BROMIDE
| InChIKey | ZASWJUOMEGBQCQ-UHFFFAOYSA-L |
LEAD(II) BROMIDE manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Lead(II) bromide CAS 10031-22-8View Details
Lead(II) bromide CAS 10031-22-8View Details
10031-22-8 -
 Lead(II) bromide CAS 10031-22-8View Details
Lead(II) bromide CAS 10031-22-8View Details
10031-22-8 -
 Lead(II) bromide CAS 10031-22-8View Details
Lead(II) bromide CAS 10031-22-8View Details
10031-22-8 -
 Lead(II) bromide CAS 10031-22-8View Details
Lead(II) bromide CAS 10031-22-8View Details
10031-22-8 -
 Lead(II) bromide CAS 10031-22-8View Details
Lead(II) bromide CAS 10031-22-8View Details
10031-22-8 -
 LEAD BROMIDE 99%View Details
LEAD BROMIDE 99%View Details
10031-22-8 -
 Lead BromideView Details
Lead BromideView Details
10031-22-8 -
 Lead BromideView Details
Lead BromideView Details
10031-22-8
