Lead dioxide
Synonym(s):Lead (su)peroxide;Lead dioxide;Lead peroxide
- CAS NO.:1309-60-0
- Empirical Formula: O2Pb
- Molecular Weight: 239.2
- MDL number: MFCD00011165
- EINECS: 215-174-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 14:24:45
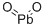
What is Lead dioxide?
Description
Lead dioxide, PbO2, also plumbic oxide, is an odorless dark-brown crystalline powder which is nearly insoluble in water. It exists in two crystalline forms. The a phase has orthorhombic symmetry, lattice constants a=0.497 nm, b=0.596 nm, c= 0.544 nm, Z=4 (four formula units per unit cell).
Chemical properties
brown to black powder
Chemical properties
Lead dioxide is a dark brown crystalline solid or powder.
Physical properties
Red tetragonal crystals or brown powder; density 9.64 g/cm3; decomposes on heating at 290°C; practically insoluble in water; also insoluble in alkalis; moderately soluble in hydrochloric acid and also, in nitric acid-hydrogen peroxide mixture; slowly dissolves in acetic acid.
Occurrence
Lead dioxide occurs in nature as the mineral plattnerite. It is used as an oxidizing agent in manufacturing dyes and intermediates. It also is used as a source of oxygen in matches, pyrotechnics, and explosives. In matches, the oxide is combined with amorphous phosphorus as an ignition surface. It also is used in making lead pigments, liquid polysulfide polymers and rubber substitutes. Lead dioxide electrodes are used in lead storage batteries in which lead dioxide accumulates on positive plates.
The Uses of Lead dioxide
Lead dioxide occurs in nature as the mineral plattnerite. It is used as an oxidizing agent in manufacturing dyes and intermediates. It also is used as a source of oxygen in matches, pyrotechnics, and explosives. In matches, the oxide is combined with amorphous phosphorus as an ignition surface. It also is used in making lead pigments, liquid polysulfide polymers and rubber substitutes. Lead dioxide electrodes are used in lead storage batteries in which lead dioxide accumulates on positive plates.
The Uses of Lead dioxide
The Uses of Lead dioxide
Lead dioxide is a strong oxidizing agent that is used in the manufacture of matches, pyrotechnics, dyes and other chemicals. It also has several important applications in the electrochemical industry, in particular as a component of lead–acid batteries used in almost all types of vehicles.
Preparation
Lead dioxide is produced by oxidizing an alkaline slurry of lead monoxide with chlorine, sodium hypochlorite, or bleaching powder. Alternatively, it is obtained by passing chlorine into a hot aqueous suspension of lead sulfate and magnesium hydroxide. The ionic reaction is:
Pb(OH)3ˉ +ClOˉ → PbO2 + Clˉ+ OHˉ + H2O
It also is produced by electrolysis of acidic solutions of lead salts using a lead or platinum electrode. In such electrolytic process, lead dioxide is deposited on the anode of the cell.
Insoluble powdered lead dioxide also may be obtained when lead tetroxide is heated with nitric acid:
Pb3 O4 + 4HNO3 → 2Pb(N)3)2 + PbO2 + 2H2O
Lead dioxide also can be prepared by fusing lead monoxide with a mixture of sodium nitrate and sodium chlorate.
General Description
Brown, hexagonal crystals. Insoluble in water. Used in matches, explosives, electrodes.
Reactivity Profile
Noncombustible but accelerates the burning of combustible material. Reacts violently with hydrogen sulfide [Bretherick 1979. p. 977-978]. Ignites with hydroxylamine [Mellor 8:291. 1946-47]. Reacts violently with hydrogen peroxide [Mellor 1:937 1946-47], with phenylhydrazine [Mellor 7:637 1946-47], or with sulfuryl chloride [Mellor 10:676. 1946-47]. Reacts with incandescence with sulfur dioxide [Mellor, 1941, Vol. 7, 689]. Explodes when ground with boron or yellow phosphorus [Mellor, 1946, Vol. 5, 17]. Mixtures with sulfur and red phosphorus ignite [Mellor, 1941, Vol. 7, 689]. Reacts vigorously when heated with calcium sulfide, strontium sulfide or barium sulfide [Mellor, 1941, Vol. 3, 745].
Health Hazard
Toxic by ingestion. Inhalation of dust is toxic. Fire may produce irritating, corrosive and/or toxic gases. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. May explode from heat or contamination. Some may burn rapidly. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Potential Exposure
This material is used in electrodes for lead-acid batteries; in matches; explosives, and as a curing agent for polysulfide elastomers
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Antidotes and special procedures for lead: Persons with significant lead poisoning are sometimes treated with CaEDTA while hospitalized. This “chelating” drug causes arush of lead from the body organs into the blood and kidneys, and thus has its own hazards, and must be administered only by highly experienced medical personnel undercontrolled conditions and careful observation. Ca EDTA or similar drugs should never be used to prevent poisoningwhile exposure continues or without strict exposure control,as severe kidney damage can result.Note to physician: For severe poisoning BAL [BritishAnti-Lewisite, dimercaprol, dithiopropanol (C3H8OS2)] hasbeen used to treat toxic symptoms of certain heavy metalspoisoning. In the case of lead poisoning it may haveSOME value. Although BAL is reported to have a largemargin of safety, caution must be exercised, because toxiceffects may be caused by excessive dosage. Most can beprevented by premedication with 1-ephedrine sulfate(CAS: 134-72-5).
Storage
Color Code—Yellow: Reactive Hazard; Store in alocation separate from other materials, especially flammables and combustibles. Prior to working with this chemical you should be trained on its proper handling andstorage. Lead dioxide must be stored to avoid contact withoxidizers (such as perchlorates, peroxides, permanganates,chlorates and nitrates) chemically active metals (such aspotassium, sodium, magnesium and zinc), since violentreactions occur. Store in tightly closed containers in a cool,well-ventilated area away from combustible materials, suchas wood, paper, and oil. Lead dioxide is regulated byOSHA Standard 1910.1025. All requirements of the standard must be followed. See OSHA Standard 1910.104 andNFPA 43A Code for the Storage of Liquid and SolidOxidizers for detailed handling and storage regulations.
Shipping
UN1872 Lead dioxide, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Incompatibilities
Lead dioxide is a powerful oxidizer. Violent reaction with many compounds, including reducing agents; chemically active metals; combustible materials, strong acids, alkaline earth sulfides, aluminum carbides, aluminum, amines, calcium sulfide, carbides, chlorine trifluoride, glycerin, hydrides, hydrochloric acid, hydrogen peroxide, hydrogen sulfide, hydroxylamine, magnesium, metal powders, metal sulfides, molybdenum, phenylhydrazine, phosphorous red/friction, phosphorous trichloride, silicon, sulfides, sulfur, sulfur dioxide, sulfur/friction, sulfuric acid, tungsten, hydrogen trisulfide
Waste Disposal
Conversion to soluble salt, precipitation as sulfide and return to supplier. Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Lead dioxide
| Melting point: | 290 °C |
| Density | 9,38 g/cm3 |
| form | Powder |
| color | Brown to black |
| Specific Gravity | 9.38 |
| Water Solubility | Insoluble |
| Merck | 14,5407 |
| Exposure limits | ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| CAS DataBase Reference | 1309-60-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Lead dioxide(1309-60-0) |
| EPA Substance Registry System | Lead dioxide (1309-60-0) |
Safety information for Lead dioxide
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Lead dioxide
Lead dioxide manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
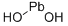
You may like
-
 Lead(IV) oxide CAS 1309-60-0View Details
Lead(IV) oxide CAS 1309-60-0View Details
1309-60-0 -
 Lead(IV) oxide CAS 1309-60-0View Details
Lead(IV) oxide CAS 1309-60-0View Details
1309-60-0 -
 Lead(IV) oxide CAS 1309-60-0View Details
Lead(IV) oxide CAS 1309-60-0View Details
1309-60-0 -
 Lead(IV) oxide CAS 1309-60-0View Details
Lead(IV) oxide CAS 1309-60-0View Details
1309-60-0 -
 Lead Dioxide (Lead Peroxide) extrapure CAS 1309-60-0View Details
Lead Dioxide (Lead Peroxide) extrapure CAS 1309-60-0View Details
1309-60-0 -
 Lead(IV) oxide CAS 1309-60-0View Details
Lead(IV) oxide CAS 1309-60-0View Details
1309-60-0 -
 Lead Dioxide .View Details
Lead Dioxide .View Details
1309-60-0 -
 Lead Dioxide BlackView Details
Lead Dioxide BlackView Details
1309-60-0
