Lead monoxide
Synonym(s):Lead monoxide;Lead(II) oxide
- CAS NO.:1317-36-8
- Empirical Formula: OPb
- Molecular Weight: 223.1994
- MDL number: MFCD00011164
- EINECS: 215-267-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
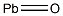
What is Lead monoxide?
Description
Lead(II) oxide occurs in two polymorphs, red, having a tetragonal crystal structure and yellow, having an orthorhombic crystal structure. Both forms occur naturally as rare minerals. The red form is known as “Litharge” and the yellow form is known as “Massico”.
Chemical properties
Lead monoxide, litharge, PbO, exists in a reddish alpha form up to 489 °C; it then transforms to a yellow beta form (massicot), which is stable at high temperatures. It has a water solubility of 17 mg/L at 20 °C, and is soluble in nitric acid, alkalies, lead acetate, ammonium chloride, and chlorides of calcium and strontium. In alkalies, it forms the plumbite ion, [PbO2]2? . Lead oxides are produced industrially by thermal processes in which lead is directly oxidized with air. In the ball mill process, metallic lead balls are tumbled in air to produce a “leady” oxide, which typically contains 20-35% free lead. The Barton pot process oxidizes droplets of molten lead at ca. 430°C to produce either litharge or leady litharge.
Physical properties
The oxide exhibits two crystalline modifications, the reddish or orange-red alpha form, known as litharge, and the yellow beta form, massicot. The alpha form constitutes tetragonal crystals while the beta modification is a yellow amorphous powder of orthorhombic crystal structure. The alpha form is stable at ordinary temperatures, converting to the beta form when heated at 489°C; density 9.35 g/cm3 (beta form); Moh’s hardness 2 (alpha form); the oxide melts at 888°C; vaporizes at 1,472°C with decomposition; vapor pressure 1 torr at 943°C and 5 torr at 1,039°C; practically insoluble in water (the solubility of alpha form is 17 mg/L at 20°C and that of beta form 23 mg/L at 22°C); insoluble in ethanol; soluble in dilute nitric acid and alkalies.
The Uses of Lead monoxide
Lead(II) oxide is employed mostly in lead-based industrial glass and industrial ceramics, including computer components. It is used as an intermediate/precursor in the manufacture of several products, for example water proof cements, lubricants, lubricating oils, inorganic pigments, lead soaps, petroleum refining, rubber, cathode ray tube glass, and polyvinyl chloride (PVC). It is useful for lead acid batteries as cathode and anode. Lead monoxide scores significant applications in oil, gas and chemical manufactures. It is an efficient catalyst for condensation reactions in organic synthesis.
The Uses of Lead monoxide
In ointments, plasters; preparing solution of lead subacetate. Glazing pottery; glass flux for painting on porcelain and glass; lead glass; varnishes; with glycerol as metal cement; producing iridescent colors on brass and bronze; coloring sulfur-containing substances, e.g., hair, nails, wool, horn; manufacture of artificial tortoise shell and horn; pigment for rubber; manufacture of boiled linseed oil; in assay of gold and silver ores.
Production Methods
Lead monoxide is obtained commercially by two processes, Barton process and the Ball Mill process. The Ball-Mill process involves reaction of molten lead with oxygen or air, and in the Barton process atomized molten lead is stirred in a mechanical furnace above 550°C. The molten metal splashed by the stirring paddle comes in contact with air fed into the cover of the furnace through a pipe, thus forming a mist of finely divided lead monoxide. The mist also contains a small amount of unreacted lead. The mist is passed through an upright shaft where a major portion of unreacted lead falls back into the furnace. It is then rapidly cooled and collected in condensing chambers. The crude product may contain 1 to 3% lead. It is finely ground and sold. The remaining lead in the crude product may be converted into the lead monoxide by stirring the molten mass in presence of air for several hours. The hot product is then cooled rapidly to a temperature below 300°C to prevent any formation of lead tetroxide, Pb3O4.
In an alternate process, a variation of the above method, molten lead is atomized in a shaft furnace. An air stream carries the very finely divided metal into the hot zone of the shaft furnace where the metal evaporates and oxidizes producing very finely divided lead monoxide. The product is passed through the cold zone of the furnace and cooled rapidly. The product obtained is a yellow powdery material, the beta form of lead oxide, massicot, consisting of orthorhombic crystals.
The red lead oxide (the tetragonal alpha modification) is obtained by slow cooling of the lead monoxide melt. The solidified mass may contain the red alpha form of the oxide resulting from slow cooling of the melt, under an outer layer of yellow beta form that may result from the rapid cooling of the outer portion.
Lead monoxide also is produced by a modified Ball Mill process in which high purity lead balls placed in the mill are partially oxidized to produce black or grey oxide. Both the red and yellow form of the oxide may be prepared by alkaline dehydration of lead hydroxide, Pb(OH)2.
General Description
Odorless gray or yellow green or red-brown solid. Sinks in water.
Reactivity Profile
Lead monoxide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. Aluminum carbide is oxidized with incandescence on warming with lead oxide, [Mellor, 1946, Vol. 5, 872]. Mixtures of lead oxide with aluminum powder(as with other metals: sodium, zirconium) give a violent explosions, [Mellor, 1946, Vol. 5, 217, 1941].
Health Hazard
General symptoms of lead poisoning (delayed). Inhalation or ingestion causes abdominal pain (lead colic), metallic taste in mouth, loss of weight, pain in muscles, and muscular weakness. Dust may irritate eyes.
Flammability and Explosibility
Not classified
Purification Methods
Higher oxides are removed by heating under vacuum at 550o with subsequent cooling under vacuum. It is red at room temperature but becomes yellow at high temperatures (~480o) reversibly. [Ray & Ogg J Am Chem Soc 78 5994 1956, Kwestroo et al. J Inorg Nucl Chem 29 39 1967.]
Properties of Lead monoxide
| Melting point: | 886 °C(lit.) |
| Boiling point: | 1470 °C |
| Density | 9.53 |
| vapor pressure | 10 mm Hg ( 0 °C) |
| refractive index | 2.67 |
| storage temp. | Store below +30°C. |
| solubility | Soluble in concentrated alkali, HCl and ammonium chloride. Insoluble in dilute alkali and alcohol. |
| form | powder |
| color | yellow |
| Specific Gravity | 9.53 |
| PH | 8-9 (100g/l, H2O, 20℃)(slurry) |
| Water Solubility | Soluble in concentrated alkali, hydrochloric acid, and ammonium chloride. Insoluble in water, dilute alkali and alcohol. |
| Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
| Merck | 14,5413 |
| Exposure limits | ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Stability: | Stable. Reacts violently with hydrogen peroxide, strong oxidizing agents, aluminium, zirconium, halogens, sulphur trioxide, boron, silicon, sodium, zinc. |
| CAS DataBase Reference | 1317-36-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Lead monoxide(1317-36-8) |
| EPA Substance Registry System | Lead monoxide (1317-36-8) |
Safety information for Lead monoxide
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H362:Reproductive toxicity, effects on or via lactation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Lead monoxide
Lead monoxide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Lead oxide, Yellow 98%View Details
Lead oxide, Yellow 98%View Details -
 Lead(II) oxide CAS 1317-36-8View Details
Lead(II) oxide CAS 1317-36-8View Details
1317-36-8 -
 Lead(II) oxide CAS 1317-36-8View Details
Lead(II) oxide CAS 1317-36-8View Details
1317-36-8 -
 Lead(II) oxide CAS 1317-36-8View Details
Lead(II) oxide CAS 1317-36-8View Details
1317-36-8 -
 Lead(II) oxide, Low silver CAS 1317-36-8View Details
Lead(II) oxide, Low silver CAS 1317-36-8View Details
1317-36-8 -
 Lead(II) oxide, Low silver CAS 1317-36-8View Details
Lead(II) oxide, Low silver CAS 1317-36-8View Details
1317-36-8 -
 Lead(II) oxide CAS 1317-36-8View Details
Lead(II) oxide CAS 1317-36-8View Details
1317-36-8 -
 Lead(II) oxide CAS 1317-36-8View Details
Lead(II) oxide CAS 1317-36-8View Details
1317-36-8
