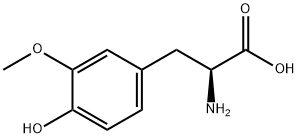
L-Tyrosine
Synonym(s):L-Tyrosine;TYR;Tyrosinase;(S)-(-)-Tyrosine;Monophenol monooxygenase
- CAS NO.:60-18-4
- Empirical Formula: C9H11NO3
- Molecular Weight: 181.19
- MDL number: MFCD00002606
- EINECS: 200-460-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
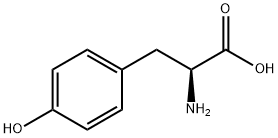
What is L-Tyrosine?
Absorption
L-tyrosine is absorbed from the small intestine by a sodium-dependent active transport process.
Toxicity
L-Tyrosine has very low toxicity. There have been very few reports of toxicity. LD50 (oral, rat) > 5110 mg/kg.
Description
L-Tyrosine is a nonessential amino acid, meaning that it is manufactured in the human body and does not need to be supplied in the diet. Under certain physiological conditions, however, it may need to be supplemented.
Like most natural amino acids, the α carbon atom in tyrosine has the L-configuration; but its enantiomer, D-tyrosine, also occurs in nature. In 1846, German chemist J. von Liebig discovered L-tyrosine in casein obtained from cheese. E. Abderhalden and Y. Teruuchi, also in Germany, isolated it from silk waste in 1906.
The body uses L-tyrosine to build proteins, many of which are involved in signal transduction. In plants, the amino acid is an electron donor in the process of photosynthesis.
Recently, V. J. J. Martin and co-workers at Concordia University (Montreal) engineered an enzyme to convert L-tyrosine to L-DOPA. This was part of a larger project to produce opiates such as codeine and morphine from yeasts, which make L-tyrosine from glucose. To aid in the enzyme screening, the research team developed a biosensor that turns L-DOPA yellow so that it biosynthesis can be tracked easily.
Chemical properties
White to off-white powder
Chemical properties
L-Tyrosine is odorless and has a bland taste. L-Tyrosine is a nonessential amino acid, as it is synthesized in the human body from phenylalanine. It is a precursor to epinephrine, norepinephrine and dopamine, three important neurotransmitters.
Occurrence
Reported found in white bread, macaroni, egg noodles, corn flakes, corn grits, oatmeal, wheat bran, wheat flakes, shredded wheat, barley brown rice, rye flour, whole grain wheat flour, buttermilk, blue cheese, cheddar cheese, cottage cheese, cream cheese, parmesan cheese, bacon, cured ham, frankfurters, pork sausage, canned red kidney beans, canned sweet corn, canned peas, canned lima beans, canned potatoes, almonds, cashews, peanuts, dates, beef, lamb, veal, chicken and turkey.
The Uses of L-Tyrosine
L-Tyrosine, one of the 22 proteinogenic amino acids essential for protein synthesis, serves as a crucial precursor for several important neurotransmitters. Derived from L-phenylalanine, it undergoes biological conversion to L-DOPA, subsequently leading to the synthesis of dopamine, norepinephrine, and epinephrine.
The Uses of L-Tyrosine
neuromuscular blocker
The Uses of L-Tyrosine
tyrosine is an amino acid. Cutaneous applications may produce an extra reserve of tyrosine in the skin, assisting or “activating” melanin synthesis. This in turn should increase and prolong the effect of the tanning process. Tyrosine’s effect is improved if the product contains vitamin B (riboflavin) plus an additional compound referred to chemically as ATP (adenosine triphosphate). experiments conducted with l-tyrosine in the form of watersoluble derivatives found that it penetrates the epidermis to the basal layer where the melanocytes are located. It is used in suntan accelerators and in skin-bronzing cosmetics to accelerate the tanning process.
Background
Tyrosine is a non-essential amino acid. In animals it is synthesized from phenylalanine. It is also the precursor of epinephrine, thyroid hormones, and melanin.
Indications
Tyrosine is claimed to act as an effective antidepressant, however results are mixed. Tyrosine has also been claimed to reduce stress and combat narcolepsy and chronic fatigue, however these claims have been refuted by some studies.
Definition
ChEBI: An optically active form of tyrosine having L-configuration.
Aroma threshold values
Detection: >10 ppm
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 28, p. 673, 1980 DOI: 10.1248/cpb.28.673
Journal of the American Chemical Society, 100, p. 3559, 1978 DOI: 10.1021/ja00479a044
Tetrahedron Letters, 29, p. 3591, 1988 DOI: 10.1016/0040-4039(88)85301-2
Biochem/physiol Actions
L-Tyrosine consists of a polar side chain and is a non-essential amino acid. It is utilized by cells to synthesize proteins that are involved in signal transduction. L-Tyrosine acts as a receiver of phosphate groups that are transferred by kinases.
Safety Profile
An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
Metabolism
In the liver, L-tyrosine is involved in a number of biochemical reactions, including protein synthesis and oxidative catabolic reactions. L-tyrosine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body.
Purification Methods
Likely impurities are L-cysteine and the ammonium salt. L-Tyrosine is dissolved in dilute ammonia, then crystallised by adding dilute acetic acid to pH 5. Also, crystallise it from H2O or EtOH/H2O, and dry it at room temperature in a vacuum over P2O5. It sublimes at 235-240o/0.03mm with 99.2% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Albert Biochem J 50 690 1952, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2348-2366 1961, Beilstein 14 IV 2264.]
Properties of L-Tyrosine
| Melting point: | >300 °C (dec.) (lit.) |
| Boiling point: | 314.29°C (rough estimate) |
| alpha | -11.65 º (c=5,DIL HCL/H2O 50/50) |
| Density | 1.34 |
| refractive index | -12 ° (C=5, 1mol/L HCl) |
| FEMA | 3736 | L-TYROSINE |
| Flash point: | 176 °C |
| storage temp. | Store below +30°C. |
| solubility | 1 M HCl: 25 mg/mL |
| form | powder |
| pka | 2.2(at 25℃) |
| color | White to Pale-brown |
| PH | 6.5 (0.1g/l, H2O) |
| Odor | odorless |
| optical activity | [α]20/D 11.5±1.0°, c = 4% in 1 M HCl |
| Water Solubility | 0.45 g/L (25 ºC) |
| Merck | 14,9839 |
| JECFA Number | 1434 |
| BRN | 392441 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong reducing agents. |
| CAS DataBase Reference | 60-18-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Tyrosine(60-18-4) |
| EPA Substance Registry System | L-Tyrosine (60-18-4) |
Safety information for L-Tyrosine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for L-Tyrosine
| InChIKey | OUYCCCASQSFEME-QMMMGPOBSA-N |
L-Tyrosine manufacturer
Clickchem Research LLP
Aquigen Bio science Pvt Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 L-Tyrosine CAS 60-18-4View Details
L-Tyrosine CAS 60-18-4View Details
60-18-4 -
 L-Tyrosine CAS 60-18-4View Details
L-Tyrosine CAS 60-18-4View Details
60-18-4 -
 L-Tyrosine ExiPlus CAS 60-18-4View Details
L-Tyrosine ExiPlus CAS 60-18-4View Details
60-18-4 -
 L-Tyrosine 99% CAS 60-18-4View Details
L-Tyrosine 99% CAS 60-18-4View Details
60-18-4 -
 60-18-4 Levodopa EP Impurity B 98%View Details
60-18-4 Levodopa EP Impurity B 98%View Details
60-18-4 -
 ISOPHORONE DIISOCYANATE (4098-71-9)View Details
ISOPHORONE DIISOCYANATE (4098-71-9)View Details
4098-71-9 -
 GLR Innovations L-Tyrosine Chemical 60-18-4, 200mlView Details
GLR Innovations L-Tyrosine Chemical 60-18-4, 200mlView Details
60-18-4 -
 Bioven Ingredients L TyrosineView Details
Bioven Ingredients L TyrosineView Details
60-18-4
