L-Tryptophan
Synonym(s):L-Tryptophan;Trp;(S)-(-)-Tryptophan;L -α-Amino-3-indolepropionic acid;L -Tryptophan
- CAS NO.:73-22-3
- Empirical Formula: C11H12N2O2
- Molecular Weight: 204.23
- MDL number: MFCD00064340
- EINECS: 200-795-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
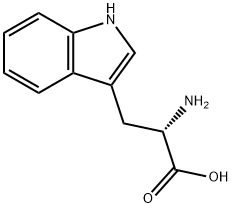
What is L-Tryptophan?
Toxicity
Oral rat LD50: > 16 gm/kg. Investigated as a tumorigen, mutagen, reproductive effector. Symptoms of overdose include agitation, confusion, diarrhea, fever, overactive reflexes, poor coordination, restlessness, shivering, sweating, talking or acting with excitement you cannot control, trembling or shaking, twitching, and vomiting.
Description
L-Tryptophan is one of nine essential amino acids for humans—“essential” in that the body cannot synthesize them, so they must be part of the diet. Tryptophan is a constituent of many common foods, especially ones that are high in protein. In the body, tryptophan is a biochemical precursor to serotonin and melatonin, a neurotransmitter and a hormone, respectively. Most commercial tryptophan is produced by microbial fermentation.
Chemical properties
White to off-white crystalline powder
Chemical properties
White crystalline powder. Odorless, slightly bitter taste; slightly soluble in water (1.14%, 25 ℃), insoluble in ethanol; soluble in dilute acid or dilute alkali. It can be colored upon long-term exposure to light. Its co-heating reaction with water will generate a small amount of indole while heating in the presence of sodium hydroxide and copper sulfate will produce a large amount of indole. It is stable upon heating together with acid in the dark, but is easily to be decomposed when heated together with other amino acids, carbohydrates and aldehydes.
The Uses of L-Tryptophan
L-tryptophan is one of the 21 amino acids comprising a protein. Tryptophan is a component of the skin’s natural moisturizing factors. It acts as a natural dietary supplement and used as an antidepressant, anxiolytic and sleep aid. It is used as a precursor to niacin, indole alkaloids and serotonin. It acts as an important intrinsic fluorescent probe, which finds to estimate the nature of microenvironment of the tryptophan.
Background
An essential amino acid that is necessary for normal growth in infants and for nitrogen balance in adults. It is a precursor of indole alkaloids in plants. It is a precursor of serotonin (hence its use as an antidepressant and sleep aid). It can be a precursor to niacin, albeit inefficiently, in mammals.
Indications
Tryptophan may be useful in increasing serotonin production, promoting healthy sleep, managing depression by enhancing mental and emotional well-being, managing pain tolerance, and managing weight.
Production Methods
The most attractive production processes for tryptophan are based on microorganisms used as enzyme sources or as overproducers: Enzymatic production from various precursors; Fermentative production from precursors; Direct fermentative production from carbohydrates by auxotrophic and analogue resistant regulatory mutants. L-tryptophan is synthesized from indole, pyruvate, and ammonia by the enzyme tryptophanase or from indole and L-serine/D,L-serine by tryptophan synthase these process variants were not economic due to the high costs of the starting materials. The microbial conversion of biosynthetic intermediates such as indole or anthranilic acid to L-tryptophan has also been considered as alternative for production.
However, the manufacturer using genetically modified strains derived fromBacillus amyloliquefaciens IAM 1521 was forced to stop Ltryptophan production. L-Tryptophan produced by this process was stigmatized because of side products found in the product causing a new severe disease termed eosinophilia-myalgia syndrome (EMS). One of the problematic impurities, "Peak E", was identified as 1,10-ethylidene- bis-(L-tryptophan), a product formed by condensation of one molecule acetaldehyde with two molecules of tryptophan.
Reactivity Profile
Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition L-Tryptophan emits toxic fumes.
Biochem/physiol Actions
Tryptophan (Trp) is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Increased Trp availability is necessary for the regulation of mood, cognition and behaviour. It is hypothesised that L-Trp might be useful in inducing sleep in healthy adults against the normal circadian rhythm. Trp uptake by the brain depends on the plasma ratio of Trp to all of the other LNAAs (large neutral amino acids). Higher the Trp:LNAAs ratio, greater is the Trp uptake.
Pharmacokinetics
Tryptophan is critical for the production of the body's proteins, enzymes and muscle tissue. It is also essential for the production of niacin, the synthesis of the neurotransmitter serotonin and melatonin. Tryptophan supplements can be used as natural relaxants to help relieve insomnia. Tryptophan can also reduce anxiety and depression and has been shown to reduce the intensity of migraine headaches. Other promising indications include the relief of chronic pain, reduction of impulsivity or mania and the treatment of obsessive or compulsive disorders. Tryptophan also appears to help the immune system and can reduce the risk of cardiac spasms. Tryptophan deficiencies may lead to coronary artery spasms. Tryptophan is used as an essential nutrient in infant formulas and intravenous feeding. Tryptophan is marketed as a prescription drug (Tryptan) for those who do not seem to respond well to conventional antidepressants. It may also be used to treat those afflicted with seasonal affective disorder (a winter-onset depression). Tryptopan serves as the precursor for the synthesis of serotonin (5-hydroxytryptamine, 5-HT) and melatonin (N-acetyl-5-methoxytryptamine).
Safety Profile
Moderately toxic by intraperitoneal route. Experimental teratogenic and reproductive effects. Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Crystallise L-tryptophan from H2O/EtOH, wash it with anhydrous diethyl ether and dry it at room temperature in a vacuum over P2O5. It sublimes at 220-230o/0.03mm with 99% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Cox & King Org Synth Coll Vol II 612 1943, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2316-2345 1961, Beilstein 22 IV 6765.]
Properties of L-Tryptophan
| Melting point: | 289-290 °C (dec.)(lit.) |
| Boiling point: | 342.72°C (rough estimate) |
| alpha | -31.1 º (c=1, H20) |
| Density | 1.34 |
| refractive index | -32 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | 20% NH3: 0.1 g/mL at 20 °C, clear, colorless |
| form | powder |
| appearance | white crystals or powder |
| pka | 2.46(at 25℃) |
| color | White to yellow-white |
| PH | 5.5-7.0 (10g/l, H2O, 20℃) |
| Odor | wh. cryst. or cryst. odorless powd., sl. bitter taste |
| optical activity | [α]20/D 31.5±1°, c = 1% in H2O |
| Water Solubility | 11.4 g/L (25 ºC) |
| Merck | 14,9797 |
| BRN | 86197 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 73-22-3(CAS DataBase Reference) |
| NIST Chemistry Reference | L-Tryptophan(73-22-3) |
| EPA Substance Registry System | L-Tryptophan (73-22-3) |
Safety information for L-Tryptophan
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H225:Flammable liquids H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P234:Keep only in original container. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P390:Absorb spillage to prevent material damage. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P370+P378:In case of fire: Use … for extinction. P405:Store locked up. P406:Store in corrosive resistant/… container with a resistant inner liner. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for L-Tryptophan
| InChIKey | QIVBCDIJIAJPQS-VIFPVBQESA-N |
L-Tryptophan manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![(1S,3S)-1-(1,3-Benzodioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid Methyl Ester](https://img.chemicalbook.in/CAS/GIF/629652-42-2.gif)


![(1S,3R)-1-Benzo[1,3]dioxol-5-yl-2-(2-chloro-acetyl)-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic Acid Methyl Ester](https://img.chemicalbook.in/CAS/GIF/629652-40-0.gif)

You may like
-
 L-tryptophan 99%View Details
L-tryptophan 99%View Details -
 L-Tryptophan 99%View Details
L-Tryptophan 99%View Details -
 L-Tryptophane CAS 73-22-3View Details
L-Tryptophane CAS 73-22-3View Details
73-22-3 -
 L-Tryptophan for tissue culture CAS 73-22-3View Details
L-Tryptophan for tissue culture CAS 73-22-3View Details
73-22-3 -
 L-Tryptophan CAS 73-22-3View Details
L-Tryptophan CAS 73-22-3View Details
73-22-3 -
 L-Tryptophan CAS 73-22-3View Details
L-Tryptophan CAS 73-22-3View Details
73-22-3 -
 L-Tryptophan ExiPlus CAS 73-22-3View Details
L-Tryptophan ExiPlus CAS 73-22-3View Details
73-22-3 -
 L-Tryptophan 99% CAS 73-22-3View Details
L-Tryptophan 99% CAS 73-22-3View Details
73-22-3
