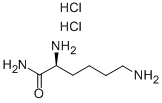
L-Lysine
Synonym(s):(S)-2,6-Diaminocaproic acid
- CAS NO.:56-87-1
- Empirical Formula: C6H14N2O2
- Molecular Weight: 146.19
- MDL number: MFCD00064433
- EINECS: 200-294-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-29 17:22:25
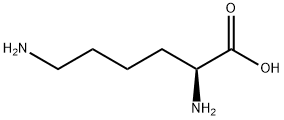
What is L-Lysine?
Absorption
Absorbed from the lumen of the small intestine into the enterocytes by an active transport process
Description
See ι-LYSINE MONOCHLORIDE.
Description
L-Lysine is one of the nine essential amino acids—essential because the human body cannot synthesize them so they must therefore be included in a healthy diet. It is one of the four common α-amino acids to have a nitrogen atom in its side chain.
In 1889, Edmund Drechsel at the University of Freiburg (Germany) isolated lysine by hydrolyzing casein, a protein found in milk. The molecule’s structure was elucidated in 1902 by Emil Fischer and Fritz Weigert at the University of Berlin; they synthesized it and compared it with the natural product.
Fortunately for humans, lysine is abundant in a wide range of high-protein foods, including red meat, some fish, eggs, cheese, and legumes. As with most amino acids, lysine’s key role in the body is the formation of proteins.
Why are we featuring lysine during National Chemistry Week? The theme for this year’s NCW is catalysis—and lysine plays a key role in a biocatalytic–organocatalytic process reported last year by Kelsey N. Stewart, Emily G. Hicks, and Dylan W. Domaille* at the Colorado School of Mines (Golden).
The researchers sought to biosynthesize value-added chemicals from renewable feedstocks by using unmodified (i.e., natural) microorganisms rather than engineered ones. They developed a process in which they combined the bacterium Gluconobacter oxidans1 and lysine as cocatalysts for the single-pot conversion of straight-chain aliphatic alcohols to α,β-unsaturated aldehydes. The reaction takes place in water under mild conditions; it is not necessary to isolate the intermediate products.
The specific role of lysine is to prevent the reaction from diverting to a simple oxidation of the starting alcohol to a carboxylic acid. The authors’ proof-of-concept reaction is the conversion of 1-butanol to 2-ethyl-2-hexenal, a chemical that is currently manufactured on a multimillion t/year scale from nonrenewable fossil-fuel feedstocks with rhodium catalysis at elevated temperature and pressure.
The authors conclude, “our study highlights that merging chemical catalysis with in situ biocatalysis is a powerful strategy to increase the complexity and breadth of products available from bioprocesses.”
1. Also known as Gluconobacter oxydans; found in spoiled (oxidized) fruit and fermented beverages.
Chemical properties
White to pale yellow crystalline powder
Chemical properties
L-Lysine is an essential amino acid (a protein building block) that cannot be produced by the body from other nutri ents. It helps ensure adequate absorption of calcium and the formation of collagen for bone, cartilage and connective tissue. This compound is odorless.
Occurrence
Some natural food sources for l-lysine include lima beans, kidney beans, potatoes, corn, red meat, fish and milk.
The Uses of L-Lysine
L-Lysine is an essential amino acid used in human nutrition. It plays a vital role in calcium absorption, building muscle protein and recovering from surgery or sports injuries. It is used for the treatment of herpes infections and cold sores. Its derivatives lysine acetylsalicylate is utilized to treat pain as well as to detoxify the body after heroin use. It is an important additive to animal feed. Further, it is used in many foods, especially red meats, fish and dairy products.
The Uses of L-Lysine
Essential amino acid for human development. Lysine residues are useful in many cellular processes, due to their ability to accept a wide variety of post-translational modifications.
The Uses of L-Lysine
A moderate serotonin antagonist and essential amino acid.
The Uses of L-Lysine
lysine is a skin-conditioning amino acid.
Background
Lysine (abbreviated as Lys or K) is an α-amino acid with the chemical formula HO2CCH(NH2)(CH2)4NH2. This amino acid is an essential amino acid, which means that humans cannot synthesize it. Its codons are AAA and AAG. Lysine is a base, as are arginine and histidine. The ε-amino group acts as a site for hydrogen binding and a general base in catalysis. Common posttranslational modifications include methylation of the ε-amino group, giving methyl-, dimethyl-, and trimethyllysine. The latter occurs in calmodulin. Other posttranslational modifications include acetylation. Collagen contains hydroxylysine which is derived from lysine by lysyl hydroxylase. O-Glycosylation of lysine residues in the endoplasmic reticulum or Golgi apparatus is used to mark certain proteins for secretion from the cell.
Indications
Supplemental lysine has putative anti-herpes simplex virus activity. There is preliminary research suggesting that it may have some anti-osteoporotic activity.
What are the applications of Application
L-Lysine is a moderate serotonin antagonist and essential amino acid
Definition
ChEBI: An L-alpha-amino acid; the L-isomer of lysine.
Preparation
Produced by fermentation. Also produced by use of continuous ion exchange technology.
Biotechnological Production
C. glutamicum and, to a lesser extent, E. coli are the main organisms used today
for industrial L-lysine production. The first L-producing strains based on C. glutamicum
were reported in 1961, and those based on E. coli in 1995. The
advantages of using E. coli versus C. glutamicum include the achievement of
higher growth rates at higher fermentation temperatures. The formation of lysine
is highly influenced by two enzymes, aspartate kinase (AK) and homoserine
dehydrogenase (HDH). AK converts aspartate into aspartate semialdehyde, and is highly feedback-inhibited by lysine and threonine. HDH converts aspartate semialdehyde
into homoserine, which is an intermediate for the biosynthesis of threonine,
methionine, and isoleucine. L-Lysine–producing strains therefore often
contain a deregulated AK and/or a reduced activity HDH. Despite the
improvement of the flux from aspartate towards lysine, the availability of key
metabolites from the central metabolic pathways is also essential. Here the formation
of oxaloacetate directly from phosphoenol pyruvate or via pyruvate is
essential for the carbon yield as some unnecessary cycles are included. For
example, inactivation of the enzyme phosphoenol pyruvate carboxykinase, which
catalyzes the reverse reaction from oxaloacetate to phosphoenol pyruvate gave an
improvement in lysine formation. By overexpression of pyruvate carboxylase,
the conversion yield of glucose to lysine could be increased by 50 %. With a
synthetic lysine hyperproducing strain, containing 12 defined modifications from
the wild type, a carbon yield of 0.55 g/g and a product titer of 120 g/L over 30 h
fermentation could be obtained.
Today, however, the main commercial process for L-lysine remains the fermentation
of C. glutamicum. This is performed in fed-batch mode in large-scale
fermenters of up to 500 m3 volume, with production capacities in excess of
100,000 tonnes. The commercial manufacturing process has been comprehensively
described by Pfefferle.
Aroma threshold values
Detection: 500 ppm
Synthesis Reference(s)
Journal of the American Chemical Society, 71, p. 3161, 1949 DOI: 10.1021/ja01177a063
General Description
L-Lysine is a basic amino acid.
Pharmacokinetics
Insures the adequate absorption of calcium; helps form collagen ( which makes up bone cartilage & connective tissues); aids in the production of antibodies, hormones & enzymes. Recent studies have shown that Lysine may be effective against herpes by improving the balance of nutrients that reduce viral growth. A deficiency may result in tiredness, inability to concentrate, irritability, bloodshot eyes, retarded growth, hair loss, anemia & reproductive problems.
Safety Profile
An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
Veterinary Drugs and Treatments
Lysine may be effective in suppressing FHV-1 infections in cats.
Metabolism
Hepatic
Purification Methods
Crystallise L-lysine from aqueous EtOH. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2097-2122 1961, Kearley & Ingersoll J Am Chem Soc 73 5783 1951, Beilstein 4 IV 2717.]
Properties of L-Lysine
| Melting point: | 215 °C (dec.)(lit.) |
| Boiling point: | 265.81°C (rough estimate) |
| alpha | D20 +14.6° (c = 6.5); D23 +25.9° (c = 2 in 6.0N HCl) |
| Density | 1.1360 (rough estimate) |
| refractive index | 26 ° (C=2, 5mol/L HCl) |
| FEMA | 3847 | L-LYSINE |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 0.1 g/mL, clear, colorless |
| form | Powder or Crystals |
| appearance | white crystals or powder |
| pka | 2.16(at 25℃) |
| color | White to light yellow |
| Odor | odorless |
| optical activity | [α]20/D +26.0±1.0°, c = 2% in 6 M HCl |
| Water Solubility | Soluble in water. Insoluble in ethanol, ethyl ether, acetone, benzene and common neutral solvent. |
| Merck | 14,5636 |
| JECFA Number | 1439 |
| BRN | 1722531 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 56-87-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Lysine(56-87-1) |
| EPA Substance Registry System | Lysine (56-87-1) |
Safety information for L-Lysine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for L-Lysine
| InChIKey | KDXKERNSBIXSRK-YFKPBYRVSA-N |
L-Lysine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 56-87-1 L-Lysine 98%View Details
56-87-1 L-Lysine 98%View Details
56-87-1 -
 L-Lysine, base CAS 56-87-1View Details
L-Lysine, base CAS 56-87-1View Details
56-87-1 -
 L-(+)-Lysine CAS 56-87-1View Details
L-(+)-Lysine CAS 56-87-1View Details
56-87-1 -
 L-Lysine (free base) Anhydrous for cell culture CAS 56-87-1View Details
L-Lysine (free base) Anhydrous for cell culture CAS 56-87-1View Details
56-87-1 -
 L-Lysine 98% CAS 56-87-1View Details
L-Lysine 98% CAS 56-87-1View Details
56-87-1 -
 L-Lysine CAS 56-87-1View Details
L-Lysine CAS 56-87-1View Details
56-87-1 -
 L-Lysine CAS 56-87-1View Details
L-Lysine CAS 56-87-1View Details
56-87-1 -
 L-Lysine CAS 56-87-1View Details
L-Lysine CAS 56-87-1View Details
56-87-1
