L-Theanine
Synonym(s):Nγ-Ethyl-L -glutamine;L -Glutamic acid γ-(ethylamide);Suntheanine
- CAS NO.:3081-61-6
- Empirical Formula: C7H14N2O3
- Molecular Weight: 174.2
- MDL number: MFCD00059653
- EINECS: 221-379-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-12 08:58:12
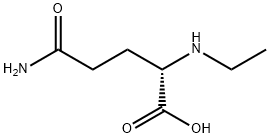
What is L-Theanine?
Description
L-Theanine is the major amino acid found in Camellia sinensis, the source of green tea. It is an analog of the excitatory neurotransmitter, glutamate, and thusly, binds to glutamate receptors. L-Theanine can antagonize various glutamate receptor subtypes as well as inhibit glutamine and glutamate transporters, which has been shown to be neuroprotective in animal models of focal cerebral ischemia. Further, L-theanine is reported to increase brain levels of dopamine, serotonin, GABA, nerve growth factor, and brain-derived neurotrophic factor.
Description
L-Theanine, or?L-2-amino-4-(ethylcarbamoyl)butyric acid, is found in tea leaves and the mushroom?Boletus badius. A May?article in the?Los Angeles Times?suggests that?L-theanine can help you improve your concentration and mental stamina. The amino acid has been added to some SoBe, Vitamin Water, and Gatorade products.
Chemical properties
White Crystalline Solid
The Uses of L-Theanine
L-Theanine is a safe and non-toxic photogenic food supplement.
L-theanine has been studied as a food additive and functional food in relation to human nutrition.
It has noticeable bioactivities including anti-cerebral ischemia-reperfusion injury, stress-reducing, antitumor, anti-aging, and anti-anxiety activities.
The Uses of L-Theanine
A non-protein amino acid mainly found naturally in the green tea plant. It may have activity in modulating the metabolism of cancer chemotherapeutics agents.
What are the applications of Application
L-Theanine is a glutamine amino acid analog
Definition
ChEBI: L-Theanine is a N(5)-alkylglutamine where the alkyl group is ethyl. It has been isolated from green tea. It has a role as a neuroprotective agent, a plant metabolite and a geroprotector. It is a tautomer of a N(5)-ethyl-L-glutamine zwitterion.
Benefits
L-Theanine is one of the active ingredients in green tea. It has a variety of physiological functions such as protecting nerves, lowering blood pressure, antioxidant, regulating immune response, improving cognitive ability and calming the mind. Therefore, L-Theanine helps to improve concentration and cognitive ability, improve immune function, reduce stress, relieve anxiety and depression symptoms, and improve sleep. In addition, studies have shown that L-Theanine also helps to alleviate symptoms in patients with high blood pressure and reduces the risk of pancreatic cancer in women.
Biological Activity
Amino acid analog of glutamine and component of green tea. Shown to bind to AMPA, Kainate, NMDA and group I mGlu receptors. Displays neuroprotective effects in vivo . Promotes self-renewal of human embryonic stem cells (hESC).
Biochem/physiol Actions
Theanine is able to bind to AMPA, kainite, and NMDA glycine receptors in rat cortical neurons but with less affinity than glutamic acid. It has been studied as a glutamate transport inhibitor, preventing glutamate uptake by M5076 ovarian sarcoma-bearing mouse cells and increasing the effect of doxorubicin on tumor growth in M5076 mice.
Side Effects
Side effects have not been reported. But drinking too much tea may cause: Headaches; Trouble staying asleep; Nausea (feeling like you are going to throw up); Irritability; Stomach pain
Synthesis
Theanine was first chemically synthesised in 1942 by Lichtenstein[28]with a yield of 90 g kg-1 by treating pyrrolidone-5-carboxylic acid with aqueous ethylamine for 20 days at 37 ℃. A number of other synthetic approaches have since been developed including a large-scale production method involving the reaction of γ-benzyl glutamate in the presence of trityl chloride and ethylamine [339 g kg-1][29]and a two-step approach involving initial dehydration of L-glutamic acid to L-pyrrolidone carboxylic acid followed by ring opening in the presence of ethylamine to yield theanine [374 g kg-1][30]. More recently, theanine was produced in four steps starting from commercially available N-phthaloyl-L-glutamic acid, which was dehydrated to the corresponding cyclic anhydride by reaction with acetic anhydride and then the ring was opened by reaction with ethylamine. Subsequent de-protection of the amine unit with hydrazine hydrate gave theanine with a 700 g kg-1 overall yield[31].
In the tea plant, theanine is bio-synthesised from glutamic acid and ethylamine by the enzyme theanine synthetase. However, the enzyme is very labile and cannot be used to produce the amino acid in commercial quantities[32]. Therefore, other methods for the enzymatic synthesis of theanine have been developed using bacterial enzymes such as glutaminase, glutamine synthetase and γ-glutamyl-transpeptidase.
Drug interactions
There is some evidence L-theanine supplements may lower blood pressure. If you take one or more medications to lower blood pressure, you may want to avoid taking L-theanine. Due to its relaxing and sleep-supporting effects, L-theanine may also have an additive effect with some sedative medications. Avoid taking supplements containing D-theanine as it may prevent the absorption of L-theanine in the body.
Source
The tropical and temperate regions of Asian, African, South American countries are considered the main origin of tea plant [C. sinensis or Thea sinensis]. It is a member of Theaceae family. The majority members of Theaceae family are obtained from India, Sri Lanka, China, and Japan. The physiological properties and colour e.g. black, white, green, yellow or oolong tea strongly depends upon the degree of fermentation and processing conditions[20]. L-theanine is mainly derived from a non-edible mushroom of Xerocomus badius, and C. sinensis. It is an amino acid which accumulates in the leaves of tea like C. sasanqua and C. japonica[21]. In tea, L-theanine is responsible for a strong smell [aroma]in general and in particular it is linked with tea umami taste[22]. From the compositional viewpoint, L-theanine comprises approximately 50% of the tea contents. Whereas, the dry tea contains 1-3% of L-theanine only, this higher or lower ratio of L-theanine can vary depending on several factors i.e. cultivation zone, production season, processing techniques, class of tea, time and type of harvest, etc[27]. Additionally, the harvested tea at the beginning of summer is reported to have more theanine compared with tea harvested in late summer[27]. Moreover, L-theanine concentration also depends on the type/class of tea. According to one study, a specific type of C. sinensis var. Sinensis has more L-theanine contents as compared to the C. sinensis var. Assamica[23].
storage
Room temperature
Properties of L-Theanine
| Melting point: | 207°C |
| Boiling point: | 430.2±40.0 °C(Predicted) |
| Density | 1.171±0.06 g/cm3(Predicted) |
| refractive index | 8 ° (C=5, H2O) |
| storage temp. | 2-8°C |
| solubility | Soluble in Water (up to 20 mg/ml). |
| form | powder |
| pka | 2.24±0.10(Predicted) |
| color | White |
| Odor | Odorless |
| Water Solubility | almost transparency |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 2 months. |
| InChI | InChI=1S/C7H14N2O3/c1-2-9-5(7(11)12)3-4-6(8)10/h5,9H,2-4H2,1H3,(H2,8,10)(H,11,12)/t5-/m0/s1 |
| CAS DataBase Reference | 3081-61-6(CAS DataBase Reference) |
Safety information for L-Theanine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for L-Theanine
| InChIKey | DATAGRPVKZEWHA-YFKPBYRVSA-N |
| SMILES | C(O)(=O)[C@H](CCC(N)=O)NCC |
L-Theanine manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 3081-61-6 L-Theanine,97% 99%View Details
3081-61-6 L-Theanine,97% 99%View Details
3081-61-6 -
 L-Theanine extrapure CAS 3081-61-6View Details
L-Theanine extrapure CAS 3081-61-6View Details
3081-61-6 -
 L-Theanine 98% CAS 3081-61-6View Details
L-Theanine 98% CAS 3081-61-6View Details
3081-61-6 -
 L-Theanine 98% (HPLC) CAS 3081-61-6View Details
L-Theanine 98% (HPLC) CAS 3081-61-6View Details
3081-61-6 -
 L-Theanine, ≥98% (HPLC) CAS 3081-61-6View Details
L-Theanine, ≥98% (HPLC) CAS 3081-61-6View Details
3081-61-6 -
 L-Theanine CAS 3081-61-6View Details
L-Theanine CAS 3081-61-6View Details
3081-61-6 -
 L-Theanine CAS 3081-61-6View Details
L-Theanine CAS 3081-61-6View Details
3081-61-6 -
 L-Theanine CAS 3081-61-6View Details
L-Theanine CAS 3081-61-6View Details
3081-61-6