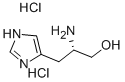
L-(-)-Histidinol dihydrochloride
Synonym(s):β-Aminoimidazole-4-propanol dihydrochloride;(S)-2-Amino-3-(4-imidazolyl)propanol dihydrochloride
- CAS NO.:1596-64-1
- Empirical Formula: C6H13Cl2N3O
- Molecular Weight: 214.09
- MDL number: MFCD00078058
- EINECS: 216-482-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
What is L-(-)-Histidinol dihydrochloride?
Description
L-Histidinol is a natural amino alcohol that serves as an intermediate in the biosynthesis of the amino acid L-histidine in bacteria, archaebacteria, fungi, and plants. It is generated from its immediate precursor, L-histidinol phosphate, by a phosphatase. L-Histidinol is oxidized to L-histidinal, which in turn is oxidized to L-histidine, by a single enzyme, histidinol dehydrogenase.
Chemical properties
white to slightly beige crystalline powder
The Uses of L-(-)-Histidinol dihydrochloride
A metabolite within Histidine metabolism.
What are the applications of Application
L-Histidinol dihydrochloride is A precursor of histamine and a reversible inhibitor of protein synthesis.
Definition
ChEBI: L-Histidinol dihydrochloride is an aralkylamine.
in vitro
preliminary experiments were done to investigate the cytotoxic effect of l-histidinol on cultured eac cells. results showed that l-histidinol up to 4 mm had no cytotoxic effects on eac cells. doxorubicin alone showed concentration-dependent cytotoxic effects. l-histidinol combination with doxorubicin led to significant potentiation of doxorubicin cytotoxicity to eac cells compared to doxorubicin alone. the concentration that caused 50% growth inhibition in eac cells after 24 h incubation was about 0.2 μg/ml, whereas it was 0.1 μg/ml when l-histidinol was added to culture medium [1].
in vivo
l-histidinol at 250 mg/kg for five consecutive doses before doxorubicin single injection could enhance the antitumour activity of doxorubicin in eac-bearing mice as demonstrated by a significant increase in average life span and cure rate of mice. in normal mice, l-histidinol, in the same dose regimen, could not alter the acute cardiotoxicity and lethality of doxorubicin [1].
References
[1] al-shabanah oa, badary oa, al-gharably nm, al-sawaf ha. effects of l-histidinol on the antitumour activity and acute cardiotoxicity of doxorubicin in mice. pharmacol res. 1998 sep;38(3):225-30.
Properties of L-(-)-Histidinol dihydrochloride
| Melting point: | 198-201 °C (dec.)(lit.) |
| alpha | -3 º (C=2, H2O 24 ºC) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Sparingly) |
| form | Crystalline Powder |
| color | White to slightly beige |
| BRN | 3914853 |
| CAS DataBase Reference | 1596-64-1(CAS DataBase Reference) |
Safety information for L-(-)-Histidinol dihydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for L-(-)-Histidinol dihydrochloride
| InChIKey | FRCAFNBBXRWXQA-XRIGFGBMSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 L-Histidinol DiHCl 95% CAS 1596-64-1View Details
L-Histidinol DiHCl 95% CAS 1596-64-1View Details
1596-64-1 -
 L-Histidinol dihydrochloride CAS 1596-64-1View Details
L-Histidinol dihydrochloride CAS 1596-64-1View Details
1596-64-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1