Janus Green B
Synonym(s):3-Diethylamino-7-(4-dimethylaminophenylazo)-5-phenylphenazinium chloride;Diazin Green S;Union Green B
- CAS NO.:2869-83-2
- Empirical Formula: C30H31ClN6
- Molecular Weight: 511.07
- MDL number: MFCD00011758
- EINECS: 220-695-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 16:37:02
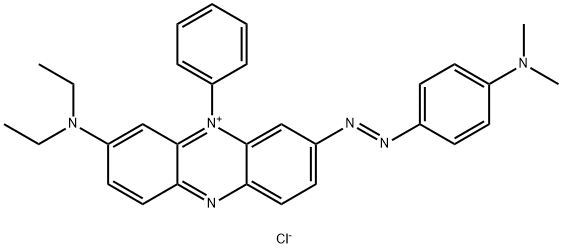
What is Janus Green B?
Chemical properties
green powder
The Uses of Janus Green B
Janus Green B is a stain that interacts with DNA and has been used for histology studies including mitochondrial staining, in which its oxidation and reduction reveals alterations to the electron transfer chain. The stain has been utilized in studies for effective and rapid staining of insect chordotonal organs and peripheral nerves, where the nerves appear dark blue in comparison to non stained tissue. With the use of Janus Green B, rabbit lymphatic vessel studies demonstrate that threadlike structures within the lymphatic vessels, known as Bonghan ducts, contain a high density of mitochondria in their cells.
The Uses of Janus Green B
As dye for cotton, wool; as biological stain; in electrodeposition of copper, Brown, Fellows, US 2882209 (1959 to Udylite Res. Corp.).
What are the applications of Application
Janus Green B is a stain used for histology studies of mitochondria
Definition
ChEBI: Janus Green B chloride is an organic chloride salt. It has a role as a dye. It contains a Janus Green B cation.
Safety Profile
Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion, skin contact, subcutaneous, and intramuscular routes. Human systemic effects by inhalation: nausea or vomiting. A skin and severe eye irritant. Flammable liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.
Purification Methods
The dye dissolves in H2O to give a bluish violet solution which becomes colourless when made 10M in NaOH. It dissolves in EtOH to give a blue-violet colour, filter from insoluble material, then add dry Et2O whereby the dye separates out leaving a small amount of blue colour in solution. Filter off the solid and dry it in a vacuum. Store it in a dark bottle. [Colour Index Vol 4, 3rd edn, 4015 1971.]
Properties of Janus Green B
| Melting point: | 160 °C (dec.)(lit.) |
| vapor pressure | 2.6 mm Hg ( 20 °C) |
| solubility | H2O: 1 M hot, clear, colorless |
| form | Powder |
| Colour Index | 11050 |
| color | ≤10(APHA) |
| Water Solubility | Soluble in water (20 mg/ml at 20°C), ethanol, and slightly soluble in alcohol. |
| λmax | 660nm, 395nm |
| Merck | 14,5255 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| Biological Applications | Antimalarialagents; diagnosis of diseases related to amyloid accumulation; diagnostic assays; detecting fungi; nucleic acids; sugars |
| CAS DataBase Reference | 2869-83-2(CAS DataBase Reference) |
| EPA Substance Registry System | Phenazinium, 3-(diethylamino)-7-[[4-(dimethylamino)phenyl]azo]-5-phenyl-, chloride (2869-83-2) |
Safety information for Janus Green B
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Janus Green B
| InChIKey | XXACTDWGHQXLGW-UHFFFAOYSA-M |
Janus Green B manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Janus green 98%View Details
Janus green 98%View Details
2869-83-2 -
 2869-83-2 99%View Details
2869-83-2 99%View Details
2869-83-2 -
 Janus Green B 95.00% CAS 2869-83-2View Details
Janus Green B 95.00% CAS 2869-83-2View Details
2869-83-2 -
 Janus Green B CAS 2869-83-2View Details
Janus Green B CAS 2869-83-2View Details
2869-83-2 -
 Janus green B CAS 2869-83-2View Details
Janus green B CAS 2869-83-2View Details
2869-83-2 -
 JANUS GREEN B For Microscopy CAS 2869-83-2View Details
JANUS GREEN B For Microscopy CAS 2869-83-2View Details
2869-83-2 -
 Janus Green B CAS 2869-83-2View Details
Janus Green B CAS 2869-83-2View Details
2869-83-2 -
 JANUS GREEN BView Details
JANUS GREEN BView Details
2869-83-2
