Isoprinosine
- CAS NO.:36703-88-5
- Empirical Formula: C24H34N6O9
- Molecular Weight: 550.57
- MDL number: MFCD05662374
- EINECS: 253-162-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
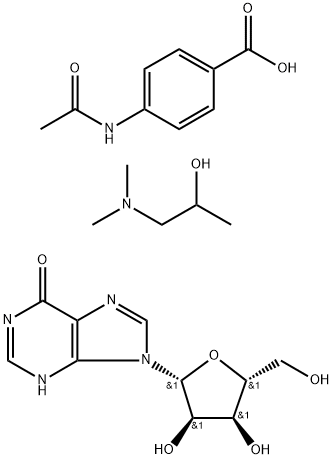
What is Isoprinosine?
Absorption
Rapidly absorbed from GIT
Toxicity
Mouse LD50 (Intravenous ): 1570 mg/kg Mouse LD50 (Oral) : 9410mg/kg Mouse LD50 (subcutaneous) ; 2960mg/kg
Chemical properties
White powder, odorless and tasteless. Soluble in water.
The Uses of Isoprinosine
Isoprinosine
The Uses of Isoprinosine
Isoprinosine is a new combination of active substances for the treatment of chronic systemic diseases such as cancer. Isoprinosine is an immunomodulator; antiviral. The ratio is between 1: 2 : 2 to 1 : 3 : 3
Indications
Inosine pranobex is also indicated for mucocutaneous infections due to herpes simplex virus (type 1 and type II) and for treatment of genital warts as adjunctive therapy to podophyllin or carbon dioxide laser.
Background
Inosine pranobex (Isoprinosine or Methisoprinol) is a combination of inosine, acetamidobenzoic acid, and dimethylaminoisopropanol used as an antiviral drug.
What are the applications of Application
Isoprinosine is an antiviral reagent
Biological Activity
isoprinosine is a complex of acetaminobenzoic acid, dimethylaminoisopropanol, and inosine in a 3:3:1 ratio with immunomodulatory effects.immunotherapy is the treatment of disease by inducing, enhancing, or suppressing an immune response. immunomodulatory regimens usually have fewer side effects than existing drugs, such as less potential for creating resistance when treating microbial disease.
Mechanism of action
Isoprinosine is thought to act through a receptor for an inosine-like compound on T cell precursors. In vivo it appears to induce the production of more mature T cells and there is some evidence that it can increase the CD4:CD8 ratio in a variety of conditions. In addition, Isoprinosine enhances B-lymphocyte activity, possibly through effects on helper T cells. It also increases macrophage phagocytosis, releases cytokines that induce macrophage proliferation, including immune interferon and interleukin-1 (IL-1) and IL-2, and potentiates T-cell mitogens.
Pharmacokinetics
Works by slowing the growth and spread of the virus in the body. It may also stimulate the immune system in the body, which helps to increase the body's ability to fight these infections.
Clinical Use
Treatment of mucocutaneous herpes simplex, genital warts, subacute sclerosing panencephalitis
in vitro
previous study found that increasing concentrations of isoprinosine (50-400 μg/ml) could produce progressively growing inhibitory effect on hhv-1 replication. the combination of 1000 iu/ml ifn-α and isoprinosine could result in enhanced anti-hhv activity [1].
in vivo
previous study demonstrated the positive effect of isoprinosine treatment on persistent infection of balb/c mice with murine gammaherpesvirus 68. increased number of leukocytes, increased percentage of neutrophils, elevated levels of virus-neutralizing antibodies, reduced number of atypical lymphocytes and reduced virus titers were detected in the examined organs after a 14-day treatment. the positive effect of isoprinosine therapy vanished after 120-150 days [2].
Metabolism
Not Available
Metabolism
Hepatically metabolised. The major excretion product of the inosine moiety is uric acid, while the p-acetamidobenzoic acid and N,N-dimethylamino- 2-propanol components are excreted in the urine as glucuronidated and oxidised products, respectively, as well as being excreted unchanged
References
[1] majewska a, lasek w, janyst m, mynarczyk g. in vitro infibition of hhv-1 replication by inosine pranobex and interferon-α. acta pol pharm. 2016 may-jun;73(3):637-44.
[2] janíková o, anicová l, briestenská k, mistríková j. the effect of isoprinosine treatment on persistent infection of balb/c mice infected with murine gammaherpesvirus 68. acta virol. 2017;61(1):32-38. doi: 10.4149/av_2017_01_32.
[3] beran j, alapová e, pajdel m; isoprinosine study (ewo iso-2014/1) team. inosine pranobex is safe and effective for the treatment of subjects with confirmed acute respiratory viral infections: analysis and subgroup analysis from a phase 4, randomised, placebo-controlled, double-blind study. bmc infect dis. 2016 nov 7;16(1):648.
Properties of Isoprinosine
| Melting point: | 140-142°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated, Sonicated), Water (Slightly |
| form | Solid |
| color | White to Off-White |
| Merck | 14,4976 |
Safety information for Isoprinosine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Isoprinosine
| InChIKey | PBJNZCQJMWVIRT-JHVGCDEENA-N |
| SMILES | O[C@@H]1[C@@H]([C@@H](CO)O[C@H]1N1C=NC2C(N=CNC1=2)=O)O.C(N(C)C)C(O)C.N(C1C=CC(C(=O)O)=CC=1)C(=O)C |&1:1,2,3,7,r| |
Isoprinosine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Inosine Pranobex 98%View Details
Inosine Pranobex 98%View Details
36703-88-5 -
 Inosine Pranobex CAS 36703-88-5View Details
Inosine Pranobex CAS 36703-88-5View Details
36703-88-5 -
 Inosine pranobex 98% (HPLC CAS 36703-88-5View Details
Inosine pranobex 98% (HPLC CAS 36703-88-5View Details
36703-88-5 -
 Inosine PranobexView Details
Inosine PranobexView Details
36703-88-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
