IPTG
Synonym(s):IPTG;Isopropyl-β-D-thiogalactopyranoside;IPTG solution;Isopropyl β-D -1-thiogalactopyranoside;Isopropyl β-D -thiogalactoside
- CAS NO.:367-93-1
- Empirical Formula: C9H18O5S
- Molecular Weight: 238.3
- MDL number: MFCD00063273
- EINECS: 206-703-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 14:33:46
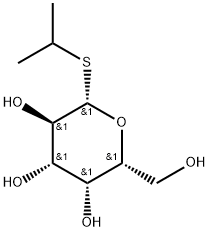
What is IPTG?
Description
IPTG (also known as Isopropyl-β-D-Thiogalactopyranoside) is a molecular biology reagent that functions as an inducer of galactosidase activity by binding to and inhibiting the repressor. It is a molecular mimic of allolactose, a lactose metabolite that triggers transcription of the lac operon, and it is therefore used to induce protein expression where the gene is under the control of the lac operator.
IPTG cannot be hydrolyzed or broken down by the E. coli therefore the concentration remains constant during cell replication. IPTG is often used at a final concentration ranging from 0.5-1.0 mM. X-Gal is often used with IPTG since it provides an easy visual method to monitor whether protein expression has occurred. X-Gal as the name implies contains a galactose group that once metabolized causes a bright blue color change.
Chemical properties
White crystalline powder
The Uses of IPTG
Isopropyl-β-D-thiogalactoside is used as a reagent in molecular biology. It is used as an effective β-galactosidase inducer of protein expression where the gene is under the control of the lac operator. It is used with 5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside to identify the non-recombinant plasmid in cloning experiments by blue-white screen methodology.
Definition
ChEBI: Isopropyl beta-D-thiogalactopyranoside is an S-glycosyl compound consisting of beta-D-1-thiogalactose having an isopropyl group attached to the anomeric sulfur.
What are the applications of Application
IPTG (Isopropyl-β-D-thiogalactopyranoside) is a galactose analogue not recognized by β-galactosidase. IPTG is a non-metabolizable galactose analog that induces expression of the lac operon in Escherichia coli. It is a commonly used reagent in cloning procedures that require induction of b-galactosidase activity and is used in conjunction with X-Gal. It is commonly used in cloning procedures that require induction of β-galactosidase activity. It is also used in conjunction with X-Gal or Bluo-Gal in blue-white selection of recombinant bacterial colonies that induce expression of the lac operon in Escherichia coli. IPTG functions by binding to the lacI repressor and altering its conformation, which prevents the repression of the β-galactosidase coding gene lacZ.
Preparation
a synthetic method of isopropyl-β-D-thiogalactoside. The steps are as follows:
Step 1: Dissolve β-D-galactose pentaacetate in any organic solvent of methylene chloride, chloroform or 1,2-dichloroethane, and add Lewis acid (boron trifluoride ether, (Aluminum trichloride, zinc chloride) catalyst and potassium ethylxanthate or sodium ethylxanthate are reacted to obtain tetraacetylgalactose ethylxanthate after treatment.
Step 2: Dissolve tetraacetyl galactose ethyl xanthate, 2-bromopropane and sodium carbonate in methanol or ethanol at a temperature of 20°C to 80°C and react for 4-6 hours to obtain isopropyl group after treatment Isopropyl-β-D-thiogalactoside. 
General Description
Inducer for β-galactosidase, an enzyme that promotes lactose utilization. Use in conjunction with X-Gal. Molecular Biology grade.
Stock solutions:
X-Gal: 20 mg/ml in DMF
IPTG: 20 mg/ml in water
Use 4:1 (X-Gal:IPTG)
Assay: ≥99%
Dioxane: None detected
Melting point: 110-114°C
Specific rotation [a]25/D (1%, water): -34.0 to -29.0°
Intended for laboratory and manufacturing use only. Not for drug, food, or household use.
Pharmacokinetics
IPTG (Isopropyl ?-D-1-thiogalactopyranoside), is a molecular biology reagent. This compound is a molecular mimic of allolactose, a lactose metabolite that triggers transcription of the lac operon and it is therefore used to induce protein expression where the gene is under the control of the lac operator.
Storage
Store powder at -20°C away from direct sunlight. Once opened and recapped, place container in a low humidity environment at the same storage temperature. Protect from moisture and light by keeping container tightly closed.
Properties of IPTG
| Melting point: | 105 °C |
| Boiling point: | 350.9°C (rough estimate) |
| alpha | -31 º (c=1, water) |
| Density | 1.3329 (rough estimate) |
| refractive index | 1.5060 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in Water or Methanol. |
| form | Crystalline Powder |
| pka | 13.00±0.70(Predicted) |
| color | White |
| Water Solubility | soluble |
| Merck | 14,5082 |
| BRN | 4631 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 367-93-1(CAS DataBase Reference) |
| EPA Substance Registry System | Isopropyl .beta.- thiogalactoside (367-93-1) |
Safety information for IPTG
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P281:Use personal protective equipment as required. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for IPTG
| InChIKey | BPHPUYQFMNQIOC-NXRLNHOXSA-N |
IPTG manufacturer
JSK Chemicals
Siri Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Isopropyl-β-(D)-thiogalactopyranoside 367-93-1 95-99 %View Details
Isopropyl-β-(D)-thiogalactopyranoside 367-93-1 95-99 %View Details
367-93-1 -
 Isopropyl-B-D-Thiogalactopyranoside (IPTG) (dioxan-free) for MB CAS 367-93-1View Details
Isopropyl-B-D-Thiogalactopyranoside (IPTG) (dioxan-free) for MB CAS 367-93-1View Details
367-93-1 -
 IPTG, solution CAS 367-93-1View Details
IPTG, solution CAS 367-93-1View Details
367-93-1 -
 Isopropyl-B-D-Thiogalactopyranoside (IPTG) (dioxan free) CAS 367-93-1View Details
Isopropyl-B-D-Thiogalactopyranoside (IPTG) (dioxan free) CAS 367-93-1View Details
367-93-1 -
 Isopropyl β-D-1-thiogalactopyranoside, for biochemistry CAS 367-93-1View Details
Isopropyl β-D-1-thiogalactopyranoside, for biochemistry CAS 367-93-1View Details
367-93-1 -
 Isopropyl-B-D-Thiogalactopyranoside (IPTG) 99% CAS 367-93-1View Details
Isopropyl-B-D-Thiogalactopyranoside (IPTG) 99% CAS 367-93-1View Details
367-93-1 -
 Isopropyl-beta-D-thiogalactopyranoside CASView Details
Isopropyl-beta-D-thiogalactopyranoside CASView Details -
 Iso propyl ß-D-1-thiogalacto pyranoside 99% Molecular Biology CAS 367-93-1View Details
Iso propyl ß-D-1-thiogalacto pyranoside 99% Molecular Biology CAS 367-93-1View Details
367-93-1
