Isoamyl acetate
Synonym(s):Acetic acid 3-methylbutyl ester;Isoamyl acetate;iso-Amyl acetate, Acetic acid isoamyl ester;Isopentyl acetate;Pentyl acetate
- CAS NO.:123-92-2
- Empirical Formula: C7H14O2
- Molecular Weight: 130.18
- MDL number: MFCD00008946
- EINECS: 204-662-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:03
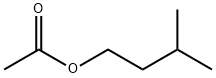
What is Isoamyl acetate?
Description
In commercial practice amyl invariably means isoamyl, unless it is prefaced by the n- for normal. Isoamyl acetate has a powerful, fruity odor with a bittersweet taste reminiscent of pear. If impure, the odor is strong, penetrating, and almost shocking. Usually prepared by esterification of commercial isoamyl alcohol with acetic acid.
Description
Isoamyl acetate is naturally produced by ripening fruit. It creates a strong, fruity banana or pear odor that is widely used to flavor foods, attract bees, and improve the smell of everything from perfumes to shoe polish. It is even used as a solvent for oil colors, lacquers, and resins; and, strangely enough, it can be used to test gas masks.
Chemical properties
Isoamyl Acetate is a strongly fruity-smelling liquid and has been identified in many fruit aromas. It is the main component of banana aroma and is, therefore, also used in banana flavors.
All isomers of amyl acetate are highly flammable, colorless to yellow, watery liquids.
Isoamyl acetate has a fruity, banana, sweet, fragrant, powerful odor with a bittersweet taste reminiscent of pear. If impure, the odor is strong, penetrating and almost shocking. In commercial practice, amyl invariably means isoamyl.
Physical properties
Clear, colorless liquid with a banana or pear-like odor. Odor threshold concentration is 7 ppm (quoted, Keith and Walters, 1992). A detection odor threshold concentration of 18 μg/m3 (3.4 ppbv) was determined by Katz and Talbert (1930).
Occurrence
Reported to be found in a number of naturally occurring products, including apple, banana, cocoa bean, coffee, cognac, grape, peach, pear, pineapple and strawberry
The Uses of Isoamyl acetate
Isoamyl acetate is used to impart pear flavorto mineral waters and syrups, in perfumes, inthe manufacture of artificial silk or leather, inphotographic films, in dyeing textiles, and asa solvent.
The Uses of Isoamyl acetate
Sting pheromone of the honeybee.1
The Uses of Isoamyl acetate
In alcohol solution as a pear flavor in mineral waters and syrups; as solvent for old oil colors, for tannins, nitrocellulose, lacquers, celluloid, and camphor; swelling bath sponges; covering unpleasant odors, perfuming shoe polish; manufacture of artificial silk, leather or pearls, photographic films, celluloid cements, waterproof varnishes, bronzing liquids, and metallic paints; dyeing and finishing textiles. A special grade of the amyl acetate has been used for burning in the Hefner lamp serving as a photometric standard.
The Uses of Isoamyl acetate
banana oil is a carrier oil. The banana family is of more interest for its nutritional value rather than for its botanical properties. The use of plantain juice as an antidote for snake bites has been reported in parts of Southeast Asia since 1916.
What are the applications of Application
Isopentyl acetate is a widely employed ester
Definition
ChEBI: The acetate ester of isoamylol.
Production Methods
The commerical-grade isoamyl acetate is prepared by the esterification of amyl alcohol (often fusel oil) with acetic acid and a small amount of sulfuric acid as the catalyst .
Preparation
By the esterification of commercial isoamyl alcohol with acetic acid
Taste threshold values
FEMA PADI: 24.491 mg
General Description
Oily liquid; colorless; banana odor. Floats and mixes with water. Flammable, irritating vapor is produced .
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Isoamyl acetate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Isoamyl acetate can react violently with oxidizing materials, nitrates, strong alkalis and strong acids.
Hazard
Flammable, moderate fire risk. Irritant. Explosive limits in air 1–7.5%.
Health Hazard
Isoamyl acetate exhibits low toxicity; thetoxic effects are comparable to those of n amyl acetate. The toxic symptoms includeirritation of the eyes, nose, and throat;fatigue; increased pulse rate; and narcosis.Inhalation of its vapors at 1000 ppm for30 minutes may cause irritation, fatigue, andrespiratory distress in humans. It is more narcotic than are the lower acetic esters. TheLD50 value in rabbits is on the order of7000 mg/kg.
Fire Hazard
FLAMMABLE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. When heated emits acrid fumes. When exposed to flames can react vigorously with reducing materials.
Flammability and Explosibility
Flammable
Potential Exposure
(n-isomer): Primary irritant (w/o allergic reaction), (sec-isomer) Human Data. Amyl acetates are used as industrial solvents and in the manufacturing and dry-cleaning industry; making artificial fruit-flavoring agents; cements, coated papers, lacquers; in medications as an inflammatory agent; pet repellents, insecticides and miticide. Many other uses.
Carcinogenicity
Not listed by ACGIH, California Proposition 65, IARC, NTP, or OSHA.
Source
Identified among 139 volatile compounds identified in cantaloupe (Cucumis melo var. reticulates cv. Sol Real) using an automated rapid headspace solid phase microextraction method (Beaulieu and Grimm, 2001).
Environmental Fate
Chemical/Physical. Slowly hydrolyzes in water forming 3-methyl-1-butanol and acetic acid.
Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
Purification Methods
Dry the acetate with finely divided K2CO3 and fractionally distil it. [Beilstein 2 IV 157.]
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrates. May soften certain plastics.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Isoamyl acetate
| Melting point: | -78 °C (lit.) |
| Boiling point: | 142 °C/756 mmHg (lit.) |
| Density | 0.876 g/mL at 25 °C (lit.) |
| vapor density | 4.5 (vs air) |
| vapor pressure | 5 mm Hg ( 25 °C) |
| refractive index | n |
| FEMA | 2055 | ISOAMYL ACETATE |
| Flash point: | 77 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | ethanol: soluble1ml/3ml, clear, colorless (60%ethanol) |
| form | neat |
| color | Clear Colorless |
| Odor | Banana-like odor |
| explosive limit | 1-10%(V) |
| Water Solubility | 0.20 g/100 mL. Slightly soluble |
| Merck | 14,5111 |
| JECFA Number | 43 |
| BRN | 1744750 |
| Henry's Law Constant | 10.25 at 37 °C (static headspace-GC, Bylaite et al., 2004) |
| Dielectric constant | 5.6(20℃) |
| Exposure limits | TLV-TWA 100 ppm (~530 mg/m3)
(ACGIH, MSHA, and OSHA); TLV-STEL
125 ppm (~655 mg/m3); IDLH 3000 ppm
(NIOSH). |
| CAS DataBase Reference | 123-92-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Butanol, 3-methyl-, acetate(123-92-2) |
| EPA Substance Registry System | Isoamyl acetate (123-92-2) |
Safety information for Isoamyl acetate
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for Isoamyl acetate
Isoamyl acetate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Isoamyl acetate 98%View Details
Isoamyl acetate 98%View Details
123-92-2 -
 Amyl Acetate pure CAS 123-92-2View Details
Amyl Acetate pure CAS 123-92-2View Details
123-92-2 -
 Isoamyl acetate, GR 99% CAS 123-92-2View Details
Isoamyl acetate, GR 99% CAS 123-92-2View Details
123-92-2 -
 Isoamyl acetate CAS 123-92-2View Details
Isoamyl acetate CAS 123-92-2View Details
123-92-2 -
 iso-Amyl acetate 98% CAS 123-92-2View Details
iso-Amyl acetate 98% CAS 123-92-2View Details
123-92-2 -
 Iso Amyl Acetate, 180 Kg, Packaging Type: DrumView Details
Iso Amyl Acetate, 180 Kg, Packaging Type: DrumView Details
123-92-2 -
 Isoamyl Acetate, For Food FlavorView Details
Isoamyl Acetate, For Food FlavorView Details
123-92-2 -
 iso-AMYL ACETATE 98% For Synthesis, For Lab Use, Grade Standard: ChemicalView Details
iso-AMYL ACETATE 98% For Synthesis, For Lab Use, Grade Standard: ChemicalView Details
123-92-2
