Allyl acetate
Synonym(s):3-Acetoxy-1-propene;Acetic acid allyl ester;Allyl acetate
- CAS NO.:591-87-7
- Empirical Formula: C5H8O2
- Molecular Weight: 100.12
- MDL number: MFCD00008721
- EINECS: 209-734-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
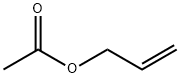
What is Allyl acetate?
Chemical properties
Allyl acetate is a flammable, colorless liquid with an acrid odor.
The Uses of Allyl acetate
Ally acetate is used in nonalcoholic beverages at 1 ppm, ice cream and ices at 2 ppm, candy at 5 ppm, baked goods at 5 ppm, and margarine at 2 ppm.
The Uses of Allyl acetate
Ally acetate is used in industrial intermediate. Used in synthesis of colophony and bond.
Production Methods
Allyl acetate is produced by the following methods: decarboxylation
of allylmalonic acid with heat, boiling ethyl 4-
chloro-N-valerate in quinoline, and acetoxylation of
propylene.
Allyl acetate is produced primarily for manufacturing allyl
alcohol. It is also used as a synthetic flavoring for
cheese, butter, and fruit.
General Description
A liquid. Insoluble in water and slightly less dense than water. Hence floats on water. Flash point below 75°F. Poisonous by ingestion and moderately toxic by inhalation and skin contact. Irritating to skin and eyes.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
Allyl acetate is an ester. Reacts with acids to liberate heat along with alcohols and acids. Generates heat with strong oxidizing acids. The reaction may be sufficiently exothermic to ignite the reaction products. Also generates heat with basic solutions. Generates flammable hydrogen with alkali metals and hydrides. Emits acrid smoke and irritating fumes when heated to decomposition.
Hazard
Skin and eye irritant; poisonous.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Inhalation or contact with some of these materials will irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Poison by ingestion. Moderately toxic by inhalation and skin contact. A skin and eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes. Dangerous fire hazard. See also ALLYL COMPOUNDS
Potential Exposure
Allyl Acetate is used to control insects in homes and animal shelters, and to treat lice in humans. May be used to enhance taste of dairy products and fruit. Generally speaking, most allyl compounds may be metabolized to allyl alcohol (see A:0540) which is metabolized to acrolein (see A:0380).
Solubility in organics
Insoluble in water, miscible with alcohol, essential oils, perfume and flavor chemicals.
Shipping
UN2333 Allyl acetate, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous materials
Purification Methods
The ester is freed from peroxides by standing with crystalline ferrous ammonium sulfate, then washed with 5% NaHCO3, followed by saturated CaCl2 solution. Dry it with Na2SO4 and fractionally distil it in an all-glass apparatus. FLAMMABLE LIQUID. [Beilstein 2 H 136, 2 IV 180.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with strong acids (liberates heat), nitrates, strong alkalis (liberates heat). Contact with alkali metals and strong reducing agents such as hydrides evolves highly flammable hydrogen gas. Attacks some plastics, coatings, and rubber. Flow or agitation of substance may generate electrostatic charges due to low conductivity; ground all equipment containing this material
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Allyl acetate
| Melting point: | 6°C |
| Boiling point: | 103-104 °C (lit.) |
| Density | 0.928 g/mL at 25 °C (lit.) |
| vapor pressure | 27.2 hPa (20 °C) |
| refractive index | n |
| Flash point: | 44 °F |
| storage temp. | 2-8°C |
| solubility | 28g/l |
| form | Liquid |
| Specific Gravity | 0.928 |
| color | Clear colorless |
| explosive limit | 2.1-13.0%(V) |
| Water Solubility | slightly |
| Merck | 14,285 |
| BRN | 1742050 |
| CAS DataBase Reference | 591-87-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, 2-propenyl ester(591-87-7) |
| EPA Substance Registry System | Acetic acid, 2-propen-1-yl ester (591-87-7) |
Safety information for Allyl acetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H301:Acute toxicity,oral H312:Acute toxicity,dermal H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Allyl acetate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Allyl acetate CAS 591-87-7View Details
Allyl acetate CAS 591-87-7View Details
591-87-7 -
 Allyl acetate CAS 591-87-7View Details
Allyl acetate CAS 591-87-7View Details
591-87-7 -
 Allyl Acetate CAS 591-87-7View Details
Allyl Acetate CAS 591-87-7View Details
591-87-7 -
 Allyl acetate CAS 591-87-7View Details
Allyl acetate CAS 591-87-7View Details
591-87-7 -
 Allyl acetate CAS 591-87-7View Details
Allyl acetate CAS 591-87-7View Details
591-87-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
