IRON (II) BROMIDE
Synonym(s):Dibromoiron;Ferrous bromide;Iron dibromide
- CAS NO.:7789-46-0
- Empirical Formula: Br2Fe
- Molecular Weight: 215.65
- MDL number: MFCD00016081
- EINECS: 232-168-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
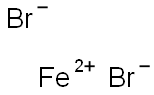
What is IRON (II) BROMIDE?
Chemical properties
Yellow to dark brown lumps
Chemical properties
In contrast to the chloride, iron(III) bromide is unstable under these conditions. The hydrated bromide can be dehydrated in a stream of hydrogen bromide at 400°C. Iron(II) bromide forms a layer lattice of the CdI2 type. It has a magnetic moment,μeff= 5·71 B.M. at 295°K. It is deliquescent and very soluble in water; the pale green hexahydrate crystallizes at room temperature, the tetrahydrate above 49°C and the dihydrate above 83°C. A 9-hydrate forms below -29·3°C. The anhydrous salt is also soluble in ether, ethanol and acetonitrile.
The Uses of IRON (II) BROMIDE
Iron(II) bromide is used as a polymerization catalyst. It is used in water treatment, chemical analysis and in ultra high purity for certain crystal growth applications.
The Uses of IRON (II) BROMIDE
Polymerization catalyst.
Preparation
Iron(II) bromide can be prepared by the direct reaction of bromine or hydrogen bromide with iron at red heat.
General Description
This product has been enhanced for catalytic efficiency.
Properties of IRON (II) BROMIDE
| Melting point: | 684 °C(lit.) |
| Boiling point: | 934 °C(lit.) |
| Density | 4.63 g/mL at 25 °C(lit.) |
| solubility | ethanol: very soluble(lit.) |
| form | beads |
| color | Yellow |
| Specific Gravity | 4.636 |
| Water Solubility | Soluble in water, THF, methanol, ethanol. |
| Sensitive | Hygroscopic |
| Merck | 14,4042 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7789-46-0(CAS DataBase Reference) |
| EPA Substance Registry System | Iron bromide (FeBr2) (7789-46-0) |
Safety information for IRON (II) BROMIDE
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P309:IF exposed or if you feel unwell: P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for IRON (II) BROMIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

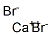
You may like
-
 Iron(II) bromide, anhydrous CAS 7789-46-0View Details
Iron(II) bromide, anhydrous CAS 7789-46-0View Details
7789-46-0 -
 Iron(II) bromide, anhydrous CAS 7789-46-0View Details
Iron(II) bromide, anhydrous CAS 7789-46-0View Details
7789-46-0 -
 Iron(II) bromide, anhydrous CAS 7789-46-0View Details
Iron(II) bromide, anhydrous CAS 7789-46-0View Details
7789-46-0 -
 Iron(II) bromide CAS 7789-46-0View Details
Iron(II) bromide CAS 7789-46-0View Details
7789-46-0 -
 Iron(II) bromide hydrate CAS 7789-46-0View Details
Iron(II) bromide hydrate CAS 7789-46-0View Details
7789-46-0 -
 Iron(II) bromide hydrate CAS 7789-46-0View Details
Iron(II) bromide hydrate CAS 7789-46-0View Details
7789-46-0 -
 Iron (II) bromide, Ultra dry CAS 7789-46-0View Details
Iron (II) bromide, Ultra dry CAS 7789-46-0View Details
7789-46-0 -
 Iron(II) bromide CAS 7789-46-0View Details
Iron(II) bromide CAS 7789-46-0View Details
7789-46-0