Iodine
- CAS NO.:7553-56-2
- Empirical Formula: I2
- Molecular Weight: 253.81
- MDL number: MFCD00011355
- EINECS: 231-442-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is Iodine?
Description
Iodine was discovered in 1811 by Bernard Courtois, and is classed among the rarer elements. Iodine is found naturally in seaweed, and is considered and generally recognized as safe substance by the US Food and Drug Administration (FDA). Iodine is a required element by many species, including humans. It has been recognized as preventative against goiter since 1819, and is used in iodized salt for this purpose. Iodine is also used as a dough oxidizer in commercial bread making. Iodine is generally extracted from natural and oil field brines by means of oxidation of iodide with chlorine, then removal from solution with an airstream. Iodine is reabsorbed in solution and reduces to hidrotic acid with sulfur dioxide. The solution is then chlorinated to precipitate free iodine, and is further purified by treatment with concentrated sulfuric acid. Iodine is the heaviest essential element for most life, with tungsten being used by some bacteria.
Description
Iodine exists mainly in its diatomic form as I2. It is a metallic gray solid which slowly sublimes to give a purple gas. Iodine is useful as a reagent for various iodination reactions. Iodine is also commonly used as a stain for visualizing compounds on TLC plates.
Chemical properties
Iodine is available as bluish-black crystals with a metallic luster and a pungent odor. It is slightly soluble in water (0.03 g/100 g). It is stable under ordinary conditions of use and storage. Iodine is incompatible with ammonia, powdered metals, alkali metals, or strong reducing agents. It reacts violently or explosively with acetaldehyde and acetylene, and reacts with ammonium hydroxide to form shock-sensitive iodides on drying. Iodine is a naturally occurring element that is essential for the good health of people and animals. Iodine is found in small amounts in seawater and in certain rocks and sediments. Iodine occurs in many different forms that can be blue, brown, yellow, red, white, or colorless. Most forms of iodine easily dissolve in water or alcohol. Iodine has many uses. Its most important use is as a disinfectant for cleaning surfaces and storage containers. It is also used in skin soaps and bandages, and for purifying water. Iodine is used in medicines and is added to food, such as table salt, to ensure that people have enough iodine in their bodies to form essential thyroid hormones. Iodine is put into animal feeds for the same reason. Iodine is used in the chemical industry for making inks and coloring agents, chemicals used in photography, and in making batteries, fuels, and lubricants. Radioactive iodine also occurs naturally. Radioactive iodine is used in medical tests and to treat certain diseases, such as over-activity or cancer of the thyroid gland. Iodine is important for the thyroid gland to produce thyroid hormones.
The Uses of Iodine

To the SM (1.77 Kg, 8.58 mol) in anhydrous DMF (8 L) under N2 at 10 C was added NIS (1.93 Kg, 8.58 mol) in portions over 10 min. The reaction mixture was stirred at RT for 2 h, after which time it was cooled using an ice bath and quenched with sat aq Na2CO3 (5 L) and extracted with EtOAc (2 x 15 L). The combined organics were washed with sat aq Na2CO3 (2 x 5 L), H2O (3 x 2 L), dried (Na2SO4), and concentrated in vacuo. The resulting crude material was purified by silica gel column chromatography (25-40% EtOAc/hexanes) to provide the product. [1.47 Kg, 57% over 2 steps]
Background
Iodine is commonly used as an antiseptic for minor cuts and abrasions, preventing infections that may result from contaminated wounds. Additionally, iodine has been studied in the treatment of fibrocystic disease and breast cancer.
Indications
Investigated for use/treatment in breast disorders (unspecified) and pain (acute or chronic).
Production Methods
In the United States, the principal method used to recover iodine from oil brines involves the oxidation of iodide by chlorine, followed by removal of the volatile iodine from solution with an airstream. The iodine is reabsorbed in solution and reduced to hidrotic acid with sulfur dioxide. The solution is then chlorinated to precipitate free iodine, which is further purified by treatment with concentrated sulfuric acid. The same process is used to recover iodine from natural brines. In the recovery of iodine from Chilean nitrate deposits, solutions containing the iodates are reduced with sodium bisulfite to precipitate the iodine, which is then purified by sublimation.
Biological Functions
Inhibition of the release of thyroid hormone by iodide is the basis for its use in hyperthyroidism. Iodide decreases the vascularity of the enlarged thyroid gland and also lowers the elevated BMR. It also has been suggested that excess iodide might change the conformation of thyroglobulin, making the protein less susceptible to thyroidal proteolysis.
Hazard
Iodine vapors are an irritant to eyes, nose and mucous membranes.Inhalation can cause headache, irritation, and congestion of lungs. Oralintake can produce burning of the mouth, vomiting, diarrhea, and abdominalcramps. Skin contact can cause rashes.
Flammability and Explosibility
Iodine is noncombustible and in itself represents a negligible fire hazard when exposed to heat or flame. However, when heated, it will increase the burning rate of combustible materials.
Environmental Fate
Iodine is released into the environment during nuclear explosions, as well as around any fuel rods, primarily spent. Due to iodine’s uses, it is frequently released into the environment, but adsorbs many minerals as well as organic masses, which inhibit transport.
storage
safety goggles and rubber gloves should be worn when handling iodine, and operations involving large quantities should be conducted in a fume hood to prevent exposure to iodine vapor or dusts by inhalation.
Toxicity evaluation
Iodine is a powerful oxidizing agent and has a direct action on cells by precipitating proteins. The affected cells may be destroyed. In addition to the primary irritant action of iodine, this compound can act as a potent sensitizer. Iodine is an integral part of thyroid hormones (tetraiodothyronine (thyroxine) and triiodothyronine), and deficiency results in compensatory hyperplasia and hypertrophy of the thyroid gland (endemic goiter). Endemic goiter occurs naturally where soil is deficient in iodine.
Properties of Iodine
| Melting point: | 113 °C (lit.) |
| Boiling point: | 184 °C (lit.) |
| Density | 1.32 g/mL at 25 °C |
| vapor density | 9 (vs air) |
| vapor pressure | 0.31 mm Hg ( 25 °C) |
| Flash point: | <10℃ |
| storage temp. | Store at RT. |
| solubility | Miscible with chloroform, carbon tetrachloride, carbon disulfide, cyclohexane, methanol, ethyl acetate, toluene, benzene, n-hexane, butan-2-ol, bromoethane, n-heptane, glycerol and diethyl ether. |
| appearance | Metallic gray solid |
| form | particles (round) |
| color | Red-brown |
| Specific Gravity | 4.93 |
| PH | 5.4 (H2O)(saturated solution) |
| Odor | Sharp, characteristic odor |
| Resistivity | 1.3E15 μΩ-cm |
| Water Solubility | 0.3 g/L (20 ºC) |
| Crystal Structure | Rhombic |
| Merck | 14,5014 |
| BRN | 3587194 |
| Exposure limits | Ceiling 0.1 ppm (~1mg/m3) (ACGIH,
MSHA, OSHA, and NIOSH); IDLH 10 ppm
(NIOSH). |
| CAS DataBase Reference | 7553-56-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Iodine(7553-56-2) |
| EPA Substance Registry System | Iodine (7553-56-2) |
Safety information for Iodine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Iodine
| InChIKey | PNDPGZBMCMUPRI-UHFFFAOYSA-N |
Iodine manufacturer
JSK Chemicals
Proto Chemicals Industries
Parad Corporation Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



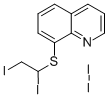
You may like
-
 Iodine, Crystalline CAS 7553-56-2View Details
Iodine, Crystalline CAS 7553-56-2View Details
7553-56-2 -
 Iodine CAS 7553-56-2View Details
Iodine CAS 7553-56-2View Details
7553-56-2 -
 Iodine CAS 7553-56-2View Details
Iodine CAS 7553-56-2View Details
7553-56-2 -
 Iodine CAS 7553-56-2View Details
Iodine CAS 7553-56-2View Details
7553-56-2 -
 Iodine CAS 7553-56-2View Details
Iodine CAS 7553-56-2View Details
7553-56-2 -
 Iodine CAS 7553-56-2View Details
Iodine CAS 7553-56-2View Details
7553-56-2 -
 Iodine CAS 7553-56-2View Details
Iodine CAS 7553-56-2View Details
7553-56-2 -
 Iodine CAS 7553-56-2View Details
Iodine CAS 7553-56-2View Details
7553-56-2