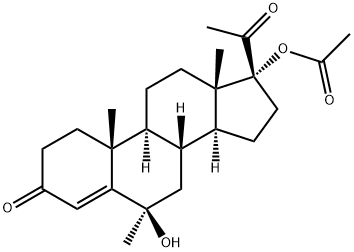
Hydroxyprogesterone caproate
Synonym(s):17α-Hydroxyprogesterone caproate;17α-Hydroxyprogesterone hexanoate
- CAS NO.:630-56-8
- Empirical Formula: C27H40O4
- Molecular Weight: 428.6
- MDL number: MFCD00072134
- EINECS: 211-138-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
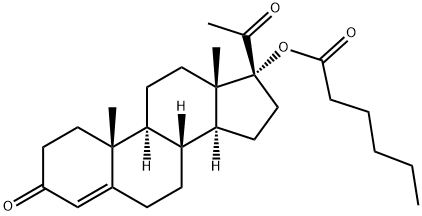
What is Hydroxyprogesterone caproate?
Absorption
Absorption of 17-hydroxyprogesteron caproate is slow, occurring over a long period of time.
Toxicity
Injection site pain is the most common adverse effect associated with hydroxyprogesterone caproate. Other commonly reported adverse effects include: injection site swelling, urticaria, pruritus, injection site pruritus, nausea, injection site nodule, and diarrhea.
Description
Hydroxyprogesterone caproate is a synthetic steroid hormone that is similar to medroxyprogesterone acetate and megestrol acetate. It is an ester derivative of 17α-hydroxyprogesterone formed from caproic acid (hexanoic acid). Hydroxyprogesterone caproate was previously marketed under the trade name Delalutin by Squibb, which was approved by the U.S. Food and Drug Administration (FDA) in 1956 and withdrawn from marketing in 1999. The U.S. FDA approved Makena from KV Pharmaceutical (previously named as Gestiva) on February 4, 2011 for prevention of preterm delivery in women with a history of preterm delivery, sparking a pricing controversy.
Originator
Delalutin,Squibb,US,1956
The Uses of Hydroxyprogesterone caproate
Hydroxyprogesterone caproate is a synthetic progestin used for the prevention of spontaneous preterm births in singleton pregnancies in women who have previously had a spontaneous preterm birth.
The Uses of Hydroxyprogesterone caproate
Hydroxyprogesterone Caproate is a synthetic progestin that reduces the chances of pre-term birth in women and improves specific fetal outcomes.
Background
Hydroxyprogesterone caproate is a synthetic steroid hormone that is similar to medroxyprogesterone acetate and megestrol acetate. It is an ester derivative of 17α-hydroxyprogesterone formed from caproic acid (hexanoic acid). Hydroxyprogesterone caproate was previously marketed under the trade name Delalutin by Squibb, which was approved by the U.S. Food and Drug Administration (FDA) in 1956 and withdrawn from marketing in 1999. The U.S. FDA approved Makena from KV Pharmaceutical (previously named as Gestiva) on February 4, 2011 for prevention of preterm delivery in women with a history of preterm delivery, sparking a pricing controversy.
In April 2023, the FDA withdrew its approval of Makena and its generics given an unfavorable risk-to-benefit assessment.
Indications
Hydroxyprogesterone caproate was previously indicated in the US for the prevention of spontaneous preterm births in singleton pregnancies in women with a history of spontaneous preterm birth. This indication was revoked by the FDA in April 2023.
Hydroxyprogesterone caproate remains indicated in other jurisdictions for the management of primary and secondary amenorrhea, corpus luteum insufficiency, and for the prevention of preterm birth.
Definition
ChEBI: Hydroxyprogesterone caproate is a corticosteroid hormone.
Manufacturing Process
40 grams of 17α-oxypregnene-(5)-ol-(3)-one-(20)-acetate-(3) is brought to
reaction with 22 grams of p-toluolsulfonic acid and 850 cc of caproic acid
anhydride under a nitrogen atmosphere for 5 days at room temperature or
2,5 days at 37°C. The excess anhydride is blown off with steam in the
presence of 200 cc of pyridine and the distillation residue is extracted with
ether and worked up as usual. The remaining oil is brought to crystallization
with pentane and the raw 17α-oxypregnenolone-3-acetate-17-caproate is
recrystallized from methanol. The crystals are needle-like and have a MP of
104° to 105°C. This substance is partially saponified by refluxing for 1 hour in
1,800 cc of methanol in the presence of 13 cc of concentrated hydrochloric
acid. After evaporation of the methanol under vacuum, the dry residue is
recrystallized from isopropyl ether or methanol (dense needles). The thus
obtained 17α-oxypregnenolone-17-caproate melts at 145° to 146.5°C.
By oxidation in 100 cc of absolute toluol with 425 cc of cyclohexanone and
155 cc of a 20% aluminum isopropylate solution in absolute toluol and after
repeated crystallizations from isopropyl ether or methanol, 24 grams of pure
17α-oxyprogesterone-17-caproate is obtained, MP 119° to 121°C (dense
needles).
Therapeutic Function
Progestin
General Description
Hydroxyprogesteronecaproate, 17-hydroxypregn-4-ene-3,20-dionehexanoate, is much more active and longer acting than progesterone, probably because the 17αester hinders reduction to the 20-ol. In contrast, hydroxyprogesteroneitself lacks progestational activity. Thecaproate ester is given only intramuscularly. The estergreatly increases oil solubility, allowing it to be slowly releasedfrom depot preparations. Although only currently available throughcompounding pharmacies, a new formulation (Gestiva) wasdeemed approvable by the FDA in late 2006 for the preventionof preterm labor, pending further studies.
Mechanism of action
Hydroxyprogesterone caproate is a synthetic progestin. Hydroxyprogesterone is a potent, long-acting, progestational steroid ester which transforms proliferative endothelium into secretory endothelium, induces mammary gland duct development, and inhibits the production and/or release of gonadotropic hormone; it also shows slight estrogenic, androgenic, or corticoid effects as well, but should not be relied upon for these effects. It's mechanism for preventing preterm birth in women with a history of preterm delivery is unknown.
Pharmacokinetics
No specific pharmacodynamic studies have been performed to assess hydroxyprogesterone caproate injections. However, the mechanism of action is likely related to increased interaction between progesterone and progesterone receptors.
Clinical Use
Hydroxyprogesterone caproate (Makena®) is used to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. Hydroxyprogesterone caproate was designated an orphan drug by the US Food and Drug Administration (FDA) for this use in 2007. Efficacy of the drug for this use is based on improvement in the proportion of women who delivered at less than 37 weeks of gestation. Direct clinical benefit (e.g., improvement in neonatal morbidity and mortality) has not been established. While there are many risk factors for preterm birth, safety and efficacy of hydroxyprogesterone caproate have been demonstrated only in women with a prior spontaneous singleton birth. Hydroxyprogesterone is not intended for use in women with multiple gestations or other risk factors for preterm birth. The American College of Obstetricians and Gynecologists (ACOG) recommends that progesterone supplementation for the prevention of recurrent preterm birth be offered to women with a singleton pregnancy and a prior spontaneous preterm birth at less than 37 weeks of gestation due to spontaneous preterm labor or premature rupture of membranes. The ACOG also states that physicians should be able to prescribe Makena or compounded hydroxyprogesterone caproate based on accepted medical indications after discussion with the patient.
Side Effects
Its side effects include: iinjection site reactions (pain, swelling, pruritus, nodule), urticaria, pruritus, nausea, diarrhea.
Safety
Originally marketed (Delalutin) nearly 50 years ago to prevent impending miscarriages, 17α-hydroxyprogesterone caproate was removed from the market in 2000 when its efficacy was called into question. 17α-Hydroxyprogesterone caproate, a synthetic injectable progesterone, was approved by the FDA in 2011 to reduce the risk of preterm delivery in women who have already experienced a preterm birth.It has not been shown to be effective in women carrying multiple fetuses.Injections begin between 16 and 21 weeks and are associated with pain, swelling, or itching at the injection site, hives, nausea, and diarrhea. Follow-up studies of the children born to women who used this drug indicate that developmental milestones were achieved at 2.5 and 5 years of age. A number of other pharmacologic agents are currently available for the prevention of preterm delivery including agents that can alter intracellular messengers (e.g., β-adrenergic receptor agonists, nitric oxide donors, magnesium sulfate and calcium channel blockers) and agents that modulate myometrial stimulants (e.g., inhibitors of prostaglandin synthesis and oxytocin antagonists).
17α-Hydroxyprogesterone caproate binds extensively to albumin and SHBG. From a metabolic perspective, it undergoes reduction, hydroxylation, and conjuga- tion reactions, including becoming glucuronidated, sulfated, and acetylated. It induces several cytochrome P450 (CYP) isozymes including CYP1A2, CYP2A6, and CYP2B6.
Metabolism
The main enzymes involved in metabolism of hydroxyprogesterone caproate are cytochrome P450 (CYP) 3A4 and to a lesser extent CYP3A5.
Properties of Hydroxyprogesterone caproate
| Melting point: | 119°C |
| alpha | +44~+50°(D/20℃)(c=1,CH3OH) |
| Boiling point: | 463.43°C (rough estimate) |
| Density | 1.0148 (rough estimate) |
| refractive index | 1.4840 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Off-White |
| Merck | 4839 |
| CAS DataBase Reference | 630-56-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Isoquinolinedione, 1,3(2h,4h)-, 2-hydroxy, acetate ester(630-56-8) |
| EPA Substance Registry System | Hydroxyprogesterone caproate (630-56-8) |
Safety information for Hydroxyprogesterone caproate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Hydroxyprogesterone caproate
| InChIKey | DOMWKUIIPQCAJU-LJHIYBGHSA-N |
| SMILES | C1(=O)C=C2[C@](C)(CC1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)[C@@](OC(=O)CCCCC)(C(=O)C)CC3)CC2 |
Hydroxyprogesterone caproate manufacturer
Add Biotec
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 630-56-8 Hydroxyprogesterone caproate impurity B 98%View Details
630-56-8 Hydroxyprogesterone caproate impurity B 98%View Details
630-56-8 -
 17-Hydroxyprogesterone caproate 630-56-8 / 2247-38-3 98%View Details
17-Hydroxyprogesterone caproate 630-56-8 / 2247-38-3 98%View Details
630-56-8 / 2247-38-3 -
 630-56-8 / 2247-38-3 17-Hydroxyprogesterone caproate 98%View Details
630-56-8 / 2247-38-3 17-Hydroxyprogesterone caproate 98%View Details
630-56-8 / 2247-38-3 -
 17-Hydroxyprogesterone caproate 98%View Details
17-Hydroxyprogesterone caproate 98%View Details
630-56-8 / 2247-38-3 -
 17α-Hydroxyprogesterone Caproate CAS 630-56-8View Details
17α-Hydroxyprogesterone Caproate CAS 630-56-8View Details
630-56-8 -
 Hydroxyprogesterone caproate CAS 630-56-8View Details
Hydroxyprogesterone caproate CAS 630-56-8View Details
630-56-8 -
 Hydroxyprogesterone Caproate Powder APIView Details
Hydroxyprogesterone Caproate Powder APIView Details
630-56-8 -
 Hydroxyprogesterone CaproateView Details
Hydroxyprogesterone CaproateView Details
630-56-8
