Hydromorphone hydrochloride
Synonym(s):(5α)-4,5-Epoxy-3-hydroxy-17-methyl-morphinan-6-one hydrochloride;Hydromorphone HCl
- CAS NO.:71-68-1
- Empirical Formula: C17H20ClNO3
- Molecular Weight: 321.8
- MDL number: MFCD03427563
- EINECS: 200-762-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
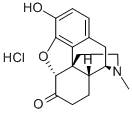
What is Hydromorphone hydrochloride?
Description
Hydromorphone hydrochloride is a pure opioid agonist with the principal therapeutic activity of analgesia. It has an analgesic potency approximately two to eight times greater than that of morphine and has a rapid onset of action. A significant feature of the analgesia is that it can occur without loss of consciousness.
Chemical properties
White Solid
The Uses of Hydromorphone hydrochloride
Analgesic (narcotic). Preparation by electrolytic reduction of Morphine. Controlled substance (opiate).
Biochem/physiol Actions
Narcotic opiate analgesic; μ opioid receptor agonist.
Clinical Use
Hydromorphone is a potent μ agonist (eight times greater than morphine) that is used to treat severe pain. It is available in intramuscular, intravenous, subcutaneous, oral, and rectal dosage forms. Like all strong μ agonists, hydromorphone is addicting and is a Schedule II drug. Hydromorphone has an oral:parenteral potency ratio of 5:1. The plasma half-lives after parenteral and oral dosage are 2.5 and 4 hours, respectively.
Safety Profile
Poison by subcutaneous and intravenous routes. Experimental teratogenic effects. A powerful analgesic. When heated to decomposition it emits very toxic fumes of NOx and HCl. See also MORPHINE.
Drug interactions
Potentially hazardous interactions with other drugs
Alcohol: can cause dose dumping with sustained
release preparations.
Analgesics: possible opioid withdrawal effects with
buprenorphine and pentazocine.
Antidepressants: possible CNS excitation or
depression with MAOIs - avoid concomitant use
and for 2 weeks after stopping MAOI; possible
CNS excitation or depression with moclobemide;
increased sedative effects with tricyclics.
Antihistamines: increased sedative effects with
sedating antihistamines.
Antipsychotics: enhanced hypotensive and sedative
effects.
Dopaminergics: avoid with selegiline.
Nalmefene: avoid concomitant use.
Sodium oxybate: enhanced effect of sodium oxybate
- avoid.
Metabolism
Hydromorphone undergoes extensive firstpass metabolism. It is extensively metabolised by glucuronidation in the liver and excreted in the urine mainly as conjugated hydromorphone, dihydroisomorphine, and dihydromorphine.
Properties of Hydromorphone hydrochloride
| Melting point: | >280°C (dec.) |
| alpha | D25 -133° |
| Density | 1.1451 (rough estimate) |
| refractive index | 1.6470 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: >10 mg/mL |
| form | A crystalline solid |
| EPA Substance Registry System | Morphinan-6-one, 4,5-epoxy-3-hydroxy-17-methyl-, hydrochloride (1:1), (5.alpha.) (71-68-1) |
Safety information for Hydromorphone hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
Computed Descriptors for Hydromorphone hydrochloride
Hydromorphone hydrochloride manufacturer
Ralington Pharma
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 71-68-1 Hydromorphone hydrochloride 98%View Details
71-68-1 Hydromorphone hydrochloride 98%View Details
71-68-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1