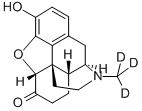
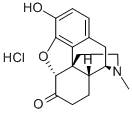
HYDROMORPHONE
- CAS NO.:466-99-9
- Empirical Formula: C17H19NO3
- Molecular Weight: 285.34
- MDL number: MFCD00864214
- EINECS: 207-383-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:03:07
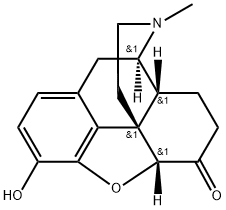
What is HYDROMORPHONE?
Absorption
The immediate release version of hydromorphone reaches its peak concentration after 30-60 minutes while the extended-release version reaches the peak concentration after 9 hours. When administered orally, hydromorphone is absorbed mainly in the upper small intestine with a bioavailability of 60% due to intensive first-pass metabolism. In the controlled-release version of hydromorphone, the absorption follows a biphasic pharmacokinetic profile. However, even though there are clear distinctions in the absorption pathway of hydromorphone, the AUC of both versions is reported to be of 34 ng.h/ml which indicates an equivalence.
The parenteral administration of hydromorphone, which is the most common pathway, presents an almost immediate absorption as observed by the presence of peak plasma concentration almost immediately. This peak plasma concentration declines rapidly due to fast redistribution into liver, spleen, kidney and skeletal muscle. In the parenteral route, the pharmacokinetic profile is log-linear and dose-dependent and to present a higher bioavailability of 78%.
Other administration routes such as rectal, nasal, intraspinal and transdermal present lower bioavailability and changes in their pharmacokinetic profile.
Toxicity
The reported LD50 of hydromorphone in the mouse was of 104 mg/kg when given intravenously and 84 mg/kg when given orally. The reports of overdose with hydromorphone are characterized by respiratory depression, somnolence, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, myosis, mydriasis, bradycardia, hypotension, apnea, circulatory collapse, cardiac arrest, and even death.
The management of an overdose might require assisted ventilation, supportive measures, as well as cardiac massage and defibrillation. It can be recommended the use of naloxone solely in the cases of respiratory depression. The use of opioid antagonist should be restricted to patients that present respiratory depression as they can produce acute abstinence symptoms.
Hydromorphone was not shown to be mutagenic nor clastogenic and long-term studies of carcinogenicity studies have not been performed. On the other hand, reduced implantation sites and viable fetuses were noted at a 2X normal concentration.
Description
Hydromorphone (Item No. 15463) is an analytical reference material categorized as an opioid. It is a metabolite of morphine (Item Nos. 15464 | ISO60147). Like other opioid analgesics, hydromorphone is commonly abused. Hydromorphone is regulated as a Schedule II compound in the United States. This product is intended for research and forensic applications.
The Uses of HYDROMORPHONE
Hydromorphone, a potent opioid, is used mainly in the palliative care seing or in patients who are not opioid naive. It can be useful if considering opioid rotation. Hydromorphone 1.3mg is approximately equianalgesic to morphine 10 mg. Both immediate- and sustained-release preparations are available.
The Uses of HYDROMORPHONE
Meperidine (pethidine) is available as parenteral and oral preparations.
There is no evidence that this opioid provides any advantage over morphine,
such as treatment of colicky-type pain. Its analgesic action is fairly short, but
the metabolite normeperidine can accumulate (t1/2 ~ 15h) if repeated doses
are given and especially if there is renal dysfunction. Normeperidine is a
CNS stimulant and can cause seizures. Its clearance is significantly reduced
in hepatic disease. Chronic use may result in enzyme induction and an
increase in normeperidine plasma concentrations. Its metabolism is
decreased by the oral contraceptive pill.
Meperidine has other significant effects related to activity at non-opioid
receptors. For example, its atropine-like action may cause a tachycardia, in
addition to direct myocardial depression at high doses. It was used originally
as a bronchodilator. It can also reduce shivering related to hypothermia or
epidural anaesthesia, although the mechanism for this is not fully
understood. Meperidine also has a local anaesthetic-like membrane
stabilising action.
Background
Hydromorphone is a pure opioid, a semi-synthetic hydrogenated ketone derivative of morphine that has been available clinically since 1920. Structurally, hydromorphone derived from morphine in the modification of the hydroxyl group in the carbon 6 to a carbonyl and the absence of a double bond between the carbon 7 and 8. Due to these modifications, it presents a very high potency and comparable side effect profile to the parent compound. Even though hydromorphone does not present a 6-hydroxyl group, it is categorized under the family of phenanthrenes and it is considered a chemical under the schedule II (medical purposes with high addiction potential).
The first reported approved product containing hydromorphone in the form of hydromorphone hydrochloride was developed by Fresenius Kabi USA and FDA approved in 1984.
Indications
Hydromorphone is indicated for the management of moderate to severe acute pain and severe chronic pain. Due to its addictive potential and overdose risk, hydromorphone is only prescribed when other first-line treatments have failed.
The WHO has proposed a three-step ladder for the management of pain in which it is suggested to start with a non-opioid medication followed by addition of weak opioids to the non-opioid treatment for moderate pain and finishing in the use of strong opioids such as hydromorphone along with the existing regimen for cases of severe pain.
Off-label, hydromorphone can be administered for the suppression of refractory cough.
Definition
ChEBI: A morphinane alkaloid that is a hydrogenated ketone derivative of morphine. A semi-synthetic drug, it is a centrally acting pain medication of the opioid class.
Biological Functions
Hydromorphone is eight times as potent as morphine but has less bioavailability following oral administration. Its side effects do not differ from those of morphine but are more intense. Hydromorphone is indicated for use in severe pain and in high doses for relief of pain in opioid-addicted patients.
General Description
Hydromorphone, (Dilaudid) is a synthetic derivative ofmorphine prepared by the catalytic hydrogenation and dehydrogenationof morphine under acidic conditions, using alarge excess of platinum or palladium. Oxidation of the 6-OH of morphine resulted in a compound with decreased potency.Reducing the 7,8 double bond of morphine increasedthe flexibility of the molecule and resulted in a compoundwith slightly enhanced binding. Making both of these structuralchanges to morphine-produced hydromorphone, acompound approximately 5 times as potent as morphine.Hydromorphone was introduced in 1926 and is available as animmediate release tablet, a liquid, and a suppository. A sustainedrelease form is available in some countries but not inthe United States. The sustained release form was removedfrom the U.S. market in 2005 when studies showed that drinking8 oz of alcohol (40%) could cause the drug to be releasedfrom the capsule immediately and lead to concentrations thatwere 5.5 times higher than in patients that did not drink alcohol.This potentially lethal combination prompted the Foodand Drug Administration (FDA) to remove it from the market.
Pharmacokinetics
In clinical trials, hydromorphone has been shown to be suitable for pain relief in patients that do not tolerate the side effects of morphine or that suffer from renal failure or asthma. It has been shown to be 5-7 times more potent than morphine with a shorter duration of analgesia.
Some of the observed effects of the consumption of hydromorphone for acute pain are complete and longlasting pain relief when compared to other pain relief agents such as meperidine, morphine, diamorphine, bupivacaine, indomethacin, and fentanyl. On the same trials, hydromorphone was shown to produce respiratory depression, lower cognitive function, miosis, mydriasis, constipation, hypotension, and vertigo but to present a reduced incidence of pruritus (which indicates a lower release of histamine) and nausea.
The respiratory depression is known to be caused by the effect on the brain stem respiratory centers as well as to a reduction in the responsiveness of this brain stems to increase carbon dioxide tension.
Metabolism
The metabolism of hydromorphone is mainly hepatic and it is represented by the generation of hydromorphone-3-glucuronide through glucuronidation reactions. This primary metabolic pathway is done by the activity of the UDP-glucuronosyltransferase-2B7. The first-pass hepatic metabolism is so large that it represents 62% of the initial administered dose.
On the other hand, hydromorphone is also characterized by the presence of minor metabolic pathways such as the CYP3A4- and CYP2C9-driven generation of norhydromorphone.
Properties of HYDROMORPHONE
| Melting point: | 266-267° |
| Boiling point: | 427.77°C (rough estimate) |
| alpha | D25 -194° (c = 0.98 in dioxane) |
| Density | 1.0864 (rough estimate) |
| refractive index | 1.5400 (estimate) |
| Flash point: | 9℃ |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Dioxane (Slightly, Sonicated) |
| form | Solid |
| pka | pKa 8.15 (Uncertain) |
| color | White to Off-White |
| Water Solubility | 1.931g/L(25 ºC) |
Safety information for HYDROMORPHONE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for HYDROMORPHONE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1