HEXAMETHONIUM BROMIDE
Synonym(s):Hexamethonium Bromide - CAS 55-97-0 - Calbiochem;Hexane-1,6-bis(trimethylammonium bromide);Hexane-1,6-bis(trimethylammonium bromide), Nicotinic Acetylcholine Receptors (nAChRs) Antagonist, Hexamethonium Bromide;N,N,N,N′,N′,N′-Hexamethylhexamethylenediammonium dibromide
- CAS NO.:55-97-0
- Empirical Formula: C12H30Br2N2
- Molecular Weight: 362.19
- MDL number: MFCD00011787
- EINECS: 200-249-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
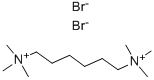
What is HEXAMETHONIUM BROMIDE?
Description
Hexamethonium is a peripherally-acting nondepolarizing neuromuscular blocking agent that acts as an antagonist of nicotinic acetylcholine receptors (nAChRs). It decreases acetylcholine release induced by carbamoylcholine in isolated cat superior cervical ganglion when used at concentrations of 27.4 and 54.8 μg/ml. It also decreases mean arterial pressure in unanesthetized rats when administered at a dose of 40 mg/kg. Intraventricular administration of hexamethonium (18 ng, i.c.v.) induces signs of nicotine abstinence, including shaking, writhing, and chewing in nicotine-dependent rats. Formulations containing hexamethonium were previously used in the treatment of hypertension.
Chemical properties
White crystalline powder and agglomerates
Originator
Bistrium,Squibb,US,1951
The Uses of HEXAMETHONIUM BROMIDE
Hexamethonium bromide has been used:
- as an autonomic ganglia-blocking agent to study its effects on whisker movement in rats
- as a nicotinic receptor antagonist to study its effects on antigen-induced arthritis (AIA) progression in mice
- as a non-selective ganglionic nicotinic acetylcholine receptor (nAChR) antagonist to study its effects on cytisine-induced autonomic cardiovascular responses in mice
What are the applications of Application
Hexamethonium bromide is a nicotinic ACh receptor antagonist
Manufacturing Process
Hexamethylene diamine (116 g), sodium carbonate (466 g), and water (800
ml) were heated to 60°C, and dimethyl sulfate (830 g) added with stirring
over 1% hours keeping the temperature below 90°C. The reaction mixture
was then stirred at 90°C for 2 hours, then cooled to 20°C, acetone (1,200 ml)
added and the whole cooled to 0°C.
The solid formed was removed by filtration and washed with acetone (150
ml). Filtrate and washings were diluted with water to 4 liters and heated to
60°C under reflux. To this was added a solution prepared from embonic acid
(388 g), sodium hydroxide (80 g) and water (5 liters), the whole refluxed for
10 minutes and thereafter allowed to cool overnight.
The resultant embonate (530 g) was filtered off, washed twice with a solution
of acetone (75 ml) in water (425 ml), and dried at 100°C to give an
amorphous yellow powder, MP 290°C to 291°C (with decomp.). 588 g of the
embonate was dissolved in boiling water (4 liters).
Hydrobromic acid 50% w/w (325 g) diluted with water (2 liters) was added
slowly at the boil and the precipitated embonic acid removed by filtering hot
and washing twice with hot water (1 liter). The filtrate and washings were
evaporated to dryness in a steam pan and the residue recrystallized from
ethyl alcohol (1,200 ml), to yield the dibromide (320 g).
Therapeutic Function
Antihypertensive
Biochem/physiol Actions
Hexamethonium bromide is a nicotinic acetylcholine receptor (nAChR) antagonist. It preferentially blocks nicotinic receptors at autonomic ganglia. It can cross the blood-brain barrier only at high doses.
Properties of HEXAMETHONIUM BROMIDE
| Melting point: | ~285 °C (dec.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Water (Slightly) |
| form | Crystalline Powder and Agglomerates |
| color | White |
| PH | pH (20g/l, 25℃) : 4.5~6.5 |
| Merck | 14,4688 |
| BRN | 3715749 |
| CAS DataBase Reference | 55-97-0(CAS DataBase Reference) |
| EPA Substance Registry System | 1,6-Hexanediaminium, N1,N1,N1,N6,N6,N6-hexamethyl-, bromide (1:2) (55-97-0) |
Safety information for HEXAMETHONIUM BROMIDE
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P405:Store locked up. |
Computed Descriptors for HEXAMETHONIUM BROMIDE
| InChIKey | FAPSXSAPXXJTOU-UHFFFAOYSA-L |
HEXAMETHONIUM BROMIDE manufacturer
Tatva Chintan Pharma Chem Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 55-97-0 Hexamethonium bromide 98%View Details
55-97-0 Hexamethonium bromide 98%View Details
55-97-0 -
 Hexamethonium Bromide CAS 55-97-0View Details
Hexamethonium Bromide CAS 55-97-0View Details
55-97-0 -
 Hexamethonium bromide CAS 55-97-0View Details
Hexamethonium bromide CAS 55-97-0View Details
55-97-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1