Gonadorelin acetate
- CAS NO.:34973-08-5
- Empirical Formula: C57H79N17O15
- Molecular Weight: 1242.36
- EINECS: 683-628-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-08 19:11:55
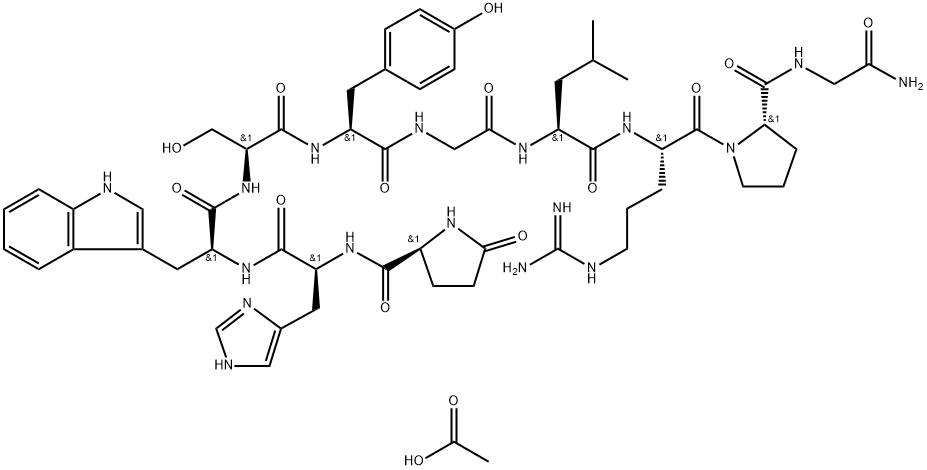
What is Gonadorelin acetate?
Description
Gonadorelin Acetate is another name for gonadotropin-releasing hormone (GnRH). It is a synthetic decapeptide prepared using solid phase peptide synthesis.
Gonadorelin, also known as GnRH or LHRH, is produced by neuroendocrine cells within the hypothalamus. The hormone is secreted pulsatilely. Gonadorelin induces the release of LH and FSH from the anterior pituitary.
After a transient increase, continuous administration of gonadorelin results in downregulation of LH and FSH levels followed by a suppression of ovarian and testicular steroid biosynthesis.
Description
Gonadorelin is a tropic hormone and agonist of the gonadotropin-releasing hormone receptor (GNRHR; Ki = 13 nM in CHO cells expressing the human receptor). It stimulates luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release from rat anterior pituitary cultures when administered at a dose of 0.1 μg. It also stimulates LH release from bovine anterior pituitary cultures at a concentration of 1 μM. In vivo, gonadorelin induces ovulation in rats pretreated with fluphenazine (; ED50 = 610.3 ng/kg). Formulations containing gonadorelin have been used as diagnostic agents to assess pituitary gland function.
The Uses of Gonadorelin acetate
Gonadorelin Acetate is used in various agricultural and biological studies. It was used in the study of ovulation synchronization in dairy cows.
The Uses of Gonadorelin acetate
Gonad-stimulating principle.
What are the applications of Application
Ovarian follicular cysts (veterinary medicine)
Use in reproduction medicine
Diagnosis of the hypothalamic-pituitary-gonadal axis function
Cryptorchism
Synchronization of ovulation (veterinary medicine)
Indications
Gonadorelin is a synthetic decapeptide with a structure identical with the natural Gonadotrophin Releasing Hormone (Gn-RH) in mammalian species.
Gonadorelin is recommended for the treatment of reproductive disorders associated with ovarian cysts and for improvement of fertility in cows and for the induction of ovulation in rabbits. The mechanism of action underlying the therapeutic use is the stimulation of the release from and also the synthesis of LH and FSH in the anterior pituitary gland.
brand name
Lutrepulse (Ferring Pharmaceuticals).
Biological Activity
Gonadorelin is a tropic hormone and agonist of the gonadotropin-releasing hormone receptor (GNRHR; Ki = 13 nM in CHO cells expressing the human receptor). It stimulates luteinizing hormone (LH) and follicle- stimulating hormone (FSH) release from rat anterior pituitary cultures when administered at a dose of 0.1 μg. It also stimulates LH release from bovine anterior pituitary cultures at a concentration of 1 μM. In vivo, gonadorelin induces ovulation in rats pretreated with fluphenazine (Item No. 23555; ED50 = 610.3 ng/kg). Formulations containing gonadorelin have been used as diagnostic agents to assess pituitary gland function.
Pharmacology
Gonadorelin is an agonist of the GnRH receptor and is used to induce the secretion of the gonadotropins follicle-stimulating hormone and luteinizing hormone from the pituitary gland and to increase sex hormone production by the gonads.
Gonadorelin has a distribution half-life of 2 to 10 minutes and a very short terminal half-life of 10 to 40 minutes. It is metabolized by hydrolysis into smaller peptide components.
Side Effects
Gonadorelin side effects: headache; flushing; nausea or abdominal discomfort; dizziness or lightheadedness; pain, swelling, or itching at the injection site; or. skin rash.
Safety Profile
A poison by intravenous route. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating vapors.
Preparation and handling
Gonadorelin (acetate) is supplied as a crystalline solid. A stock solution may be made by dissolving the gonadorelin (acetate) in the solvent of choice. Gonadorelin (acetate) is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of gonadorelin (acetate) in ethanol is approximately 0.25 mg/ml and approximately 30 mg/ml in DMSO and DMF.
Further dilutions of the stock solution into aqueous buffers or isotonic saline should be made prior to performing biological experiments. Ensure that the residual amount of organic solvent is insignificant, since organic solvents may have physiological effects at low concentrations. Organic solvent-free aqueous solutions of gonadorelin (acetate) can be prepared by directly dissolving the crystalline solid in aqueous buffers. The solubility of gonadorelin (acetate) in PBS, pH 7.2, is approximately 10 mg/ml. We do not recommend storing the aqueous solution for more than one day.
Properties of Gonadorelin acetate
| Melting point: | >155oC (dec.) |
| storage temp. | Hygroscopic, Refrigerator, Under inert atmosphere |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 34973-08-5 |
Safety information for Gonadorelin acetate
Computed Descriptors for Gonadorelin acetate
| InChIKey | NGCGMRBZPXEPOZ-NWVXUHCVNA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

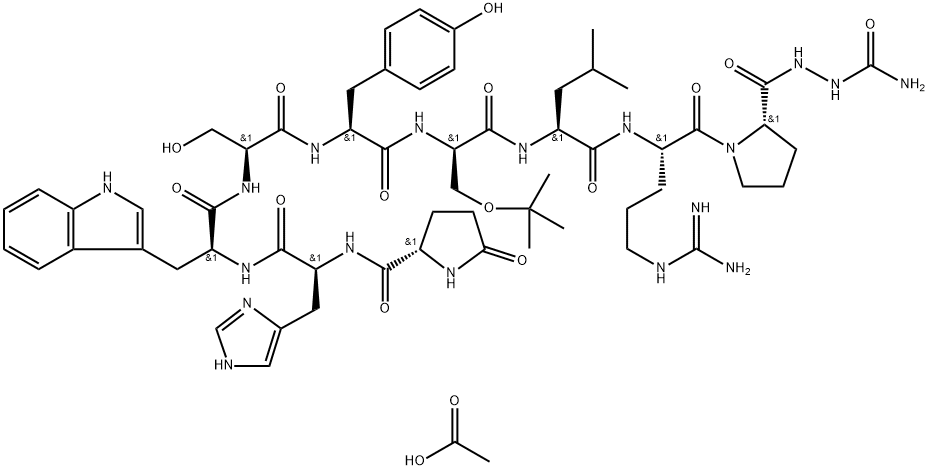
You may like
-
 Gonadorelin Acetate 2/10mg PeptideView Details
Gonadorelin Acetate 2/10mg PeptideView Details
34973-08-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
