Glycidyl phenyl ether
Synonym(s):2,3-Epoxypropyl phenyl ether;Glycidyl phenyl ether;Glycidyl phenyl ether, 1,2-Epoxy-3-phenoxypropane, Phenyl 2,3-epoxypropyl ether, Phenyl glycid ether;Phenyl glycidyl ether
- CAS NO.:122-60-1
- Empirical Formula: C9H10O2
- Molecular Weight: 150.17
- MDL number: MFCD00005133
- EINECS: 204-557-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
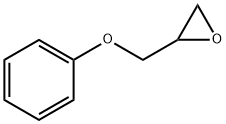
What is Glycidyl phenyl ether?
Description
This monoglycidyl derivative is a reactive diluent in epoxy resins of the bisphenol A type. It is a component of epoxy paints, epoxy glues, and epoxy resins. Sensitization was observed in many professions, such as in construction workers, marble workers, ceramic workers, and in a shoemaker.
Chemical properties
Phenyl glycidyl ether, also known as glycidyl phenyl ether or PGE, is a clear liquid with a sweet odor that is considered unpleasant. It is soluble in ether and benzene, but insoluble in water. It has the ability to volatilize with water vapor. It is used in the production of epoxy resins, as a chemical intermediate, and as a stabilizer.
The Uses of Glycidyl phenyl ether
Phenyl glycidyl ether (PGE) is used as anintermediate in organic syntheses.
The Uses of Glycidyl phenyl ether
2-Phenylglycidyl ether is an organic synthesis; chemical intermediate with high solvency for halogenated materials; reactive diluent of epoxy-resin systems; forms chemical bonds with the resin during cure and accelerates the curing process.
Production Methods
PGE is synthesized by condensation of phenol with epichlorohydrin, with subsequent dehydrochlorination with caustic to form the epoxy ring.
Definition
ChEBI: Phenyl glycidyl ether is an aromatic ether.
Synthesis Reference(s)
The Journal of Organic Chemistry, 50, p. 1784, 1985 DOI: 10.1021/jo00210a053
General Description
Colorless liquid.
Air & Water Reactions
Ethers tend to form unstable peroxides when exposed to oxygen. Ethyl, isobutyl, ethyl tert-butyl, and ethyl tert-pentyl ether are particularly hazardous in this respect. Ether peroxides can sometimes be observed as clear crystals deposited on containers or along the surface of the liquid. Slightly soluble in water.
Reactivity Profile
Glycidyl phenyl ether, an ether, can act as a base. They form salts with strong acids and addition complexes with Lewis acids. The complex between diethyl ether and boron trifluoride is an example. Ethers may react violently with strong oxidizing agents. In other reactions, which typically involve the breaking of the carbon-oxygen bond, ethers are relatively inert.
Health Hazard
PGE is a toxic compound exhibiting moderate irritant action and carcinogenicity inanimals. Application of 0.25 mg resulted insevere eye irritation in rabbits, while 500 mgcaused moderate skin irritation over a periodof 24 hours. Prolonged or repeated contactcan cause moderate irritation and skin sensitization in humans.
The symptoms of its toxicity in animalswere depression of the central nervous system and paralysis of the respiratory tract.Prolonged exposure caused changes in thekidney, liver, thymus, and testes, and lossof hair in rats. The toxicity of this compound in humans is low and the health hazardcan arise primarily from its skin-sensitizationaction.
LD50 value, oral (mice): 1400 mg/kg
DGE showed carcinogenicity in rats, causingnasal cancer.
Fire Hazard
Glycidyl phenyl ether is probably combustible.
Contact allergens
This monoglycidyl derivative is a reactive diluent in epoxy resins Bisphenol A type. It is a component of epoxy paints, epoxy glues, and epoxy resins. Sensitization has been observed in many professions, such as in construction workers, marble workers, ceramic workers, and shoemakers.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Moderately toxic by ingestion, skin contact, and subcutaneous routes. A severe eye and skin irritant. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a chemical intermediate. See also ETHERS
Potential Exposure
PGE is used to increase storage time and stability of halogenated compounds; as a reactive diluent in uncured epoxy resins to reduce the viscosity of the uncured system for ease in casting; adhesive, and laminating applications. NIOSH once estimated that 8000 workers are potentially exposed to PGE.
Carcinogenicity
Chronic exposure of rats to 1 or 12ppm 6 hours/day, 5 days/week for 2 years caused an increased incidence of rhinitis, squamous metaplasia, and epidermal carcinomas of the nasal cavity.4 The IARC has determined that there is sufficient evidence for the carcinogenicity of PGE in animals and that it is possibly carcinogenic to humans.
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, amines, and curing agents. PGE can presumably form explosive peroxides
Waste Disposal
Concentrated waste containing no peroxides-discharge liquid at a controlled rate near a pilot flame. Concentrated waste containing peroxidesperforation of a container of the waste from a safe distance followed by open burning.
Properties of Glycidyl phenyl ether
| Melting point: | 3.5 °C(lit.) |
| Boiling point: | 245-247 °C(lit.) |
| Density | 1.109 g/mL at 25 °C(lit.) |
| vapor density | 5.2 (vs air) |
| vapor pressure | 0.03 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | 2.4g/l |
| form | Liquid |
| color | Clear colorless to yellow |
| Water Solubility | 2.4 g/L (20 ºC) |
| BRN | 2744 |
| Exposure limits | TLV-TWA 6 mg/m3
(1 ppm) (ACGIH);
1 ppm (15 min) (NIOSH). |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 122-60-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Oxirane, (phenoxymethyl)-(122-60-1) |
| IARC | 2B (Vol. 47, 71) 1999 |
| EPA Substance Registry System | Phenyl glycidyl ether (122-60-1) |
Safety information for Glycidyl phenyl ether
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H341:Germ cell mutagenicity H350:Carcinogenicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Glycidyl phenyl ether
| InChIKey | FQYUMYWMJTYZTK-UHFFFAOYSA-N |
Glycidyl phenyl ether manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Phenyl Glycidyl Ether 122-60-1 98%View Details
Phenyl Glycidyl Ether 122-60-1 98%View Details
122-60-1 -
 122-60-1 Glycidyl phenyl ether 99%View Details
122-60-1 Glycidyl phenyl ether 99%View Details
122-60-1 -
 Glycidyl Phenyl Ether CAS 122-60-1View Details
Glycidyl Phenyl Ether CAS 122-60-1View Details
122-60-1 -
 1,2-Epoxy-3-phenoxypropane CAS 122-60-1View Details
1,2-Epoxy-3-phenoxypropane CAS 122-60-1View Details
122-60-1 -
 1,2-Epoxy-3-phenoxypropane CAS 122-60-1View Details
1,2-Epoxy-3-phenoxypropane CAS 122-60-1View Details
122-60-1 -
 2,3-Epoxypropyl phenyl ether CAS 122-60-1View Details
2,3-Epoxypropyl phenyl ether CAS 122-60-1View Details
122-60-1 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1